Abstract
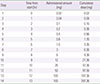
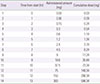
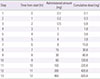
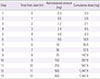
Tuberculosis is an infectious disease that can be treated using a combination of antitubercular drugs. First-line antitubercular agents such as isoniazid and rifampin are considered pivotal to successful treatment. However, they are also known to have relatively high rates of adverse events including hypersensitivity reactions. Discontinuing the first-line agents in the event of hypersensitivity may significantly compromise the cure rate of tuberculosis. Drug desensitization can be an effective method allowing continued use of the first-line agents and achieving successful cure of tuberculosis. A 70-year-old man was diagnosed with culture proven pulmonary tuberculosis and treated with first-line antitubercular agents (isoniazid, 300 mg; rifampin, 600 mg; pyrazinamide, 1,500 mg; and ethambutol, 800 mg). After 2 weeks of treatment, generalized erythematous papular rash and fever developed, for which all drugs were discontinued. Since he had hypersensitivity to all 4 first-line antitubercular agents, we tried desensitization for all 4 drugs one by one to resume antituberculosis treatment. After successful desensitization of all 4 first-line antituberculosis drugs, 6 months-antitubercular therapy was completed without any complications. We report here a case of multiple desensitization in a pulmonary tuberculosis patient having hypersensitivity to all of the 4 first-line antitubercular drugs, successfully completing 6-month antitubercular therapy without any complications.
Figures and Tables
References
2. Global tuberculosis report 2012 [Internet]. Geneva: World Health Organization;c2012. cited 2013 Jul 19. Available from: http://apps.who.int/iris/bitstream/10665/75938/1/9789241564502_eng.pdf.
3. Kobashi Y, Abe T, Shigeto E, Yano S, Kuraoka T, Oka M. Desensitization therapy for allergic reactions to antituberculous drugs. Intern Med. 2010; 49:2297–2301.

4. Schaberg T, Rebhan K, Lode H. Risk factors for side-effects of isoniazid, rifampin and pyrazinamide in patients hospitalized for pulmonary tuberculosis. Eur Respir J. 1996; 9:2026–2030.

5. Holland CL, Malasky C, Ogunkoya A, Bielory L. Rapid oral desensitization to isoniazid and rifampin. Chest. 1990; 98:1518–1519.

7. Yee D, Valiquette C, Pelletier M, Parisien I, Rocher I, Menzies D. Incidence of serious side effects from first-line antituberculosis drugs among patients treated for active tuberculosis. Am J Respir Crit Care Med. 2003; 167:1472–1477.

8. Seo HS, Min PK, Kim CW, Park JW, Jeong JH, Jang KH, et al. Two cases of rifampin desensitization in AIDS patients with rifampin hypersensitivity. J Asthma Allergy Clin Immunol. 2002; 22:119–123.
9. Kuwabara K. Anti-tuberculosis chemotherapy and management of adverse reactions. Nihon Rinsho. 2011; 69:1389–1393.
10. Kim MS, Cho YJ. Cyclosporine desensitization in patient with multiple hypersensitivity reactions immediately after peripheral blood stem cell transplantation. Korean J Asthma Allergy Clin Immunol. 2008; 28:59–63.
11. Millikan LE, Mroczkowski TF. Immunology of adverse drug eruptions. Clin Dermatol. 1986; 4:30–39.

12. Moreno-Ancillo A, Lopez-Serrano MC. Hypersensitivity reactions to drugs in HIV-infected patients. Allergic evaluation and desensitization. Clin Exp Allergy. 1998; 28:Suppl 4. 57–60.
13. Scherer K, Brockow K, Aberer W, Gooi JH, Demoly P, Romano A, et al. Desensitization in delayed drug hypersensitivity reactions: an EAACI position paper of the Drug Allergy Interest Group. Allergy. 2013; 68:844–852.

15. Asai S, Shimoda T, Hara K, Fujiwara K. Occupational asthma caused by isonicotinic acid hydrazide (INH) inhalation. J Allergy Clin Immunol. 1987; 80:578–582.

16. Thong BY. Update on the management of antibiotic allergy. Allergy Asthma Immunol Res. 2010; 2:77–86.

17. Kim JH, Kim HB, Kim BS, Hong SJ. Rapid oral desensitization to isoniazid, rifampin, and ethambutol. Allergy. 2003; 58:540–541.

18. Kobashi Y, Okimoto N, Matsushima T, Abe T, Nishimura K, Shishido S, et al. Desensitization therapy for antituberculous drugs. Kekkaku. 2000; 75:521–526.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download