Abstract
Purpose
Soluble ST2 (sST2) has been reported to regulate Th2 response. In this study, serum levels of sST2 and other cytokines were measured in recurrent early wheezers and asthmatic children. We aimed to investigate if there are any differences or similarities in Th1 or Th2 response between those two patient groups.
Methods
Fifty-nine patients admitted with exacerbation of wheezing or asthma were enrolled. Two patient groups were defined: children with atopic asthma (≥6 years, n=21) and recurrent early wheezers (≤2 years, n=38). Recurrent early wheezers were divided based on their atopic status: 19 were atopic and 19 were nonatopic. sST2, interleukin (IL) 33, IL-5, and interferon (IFN)-γ were measured in serum samples collected on admission. Cytokine levels in both patient groups were compared with their age-matched controls and evaluated the relationship with blood eosinophils, serum IgE levels, and also with the severity of symptom.
Results
sST2 and IL-5 were significantly increased both in asthmatic children (P=0.02, P=0.004) and recurrent early wheezers (P=0.01, P=0.001) compared to their age-matched controls. IL-5 was significantly higher in atopic wheezers compared with non-atopic wheezers (P=0.04). Severity score showed a positive correlation with sST2 and IFN-γ in asthmatic children, but only with IFN-γ in early wheezers. There was an inverse correlation between sST2 and blood eosinophil counts both in asthmatic children and atopic recurrent wheezers.
Figures and Tables
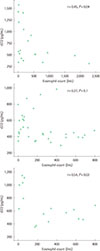 | Fig. 1Correlation between serum soluble ST2 levels and peripheral blood eosinophil counts in asthmatic children (A), recurrent early wheezers (B), and atopic wheezers (C). |
References
1. Tago K, Noda T, Hayakawa M, Iwahana H, Yanagisawa K, Yashiro T, et al. Tissue distribution and subcellular localization of a variant form of the human ST2 gene product, ST2V. Biochem Biophys Res Commun. 2001; 285:1377–1383.

2. Iwahana H, Hayakawa M, Kuroiwa K, Tago K, Yanagisawa K, Noji S, et al. Molecular cloning of the chicken ST2 gene and a novel variant form of the ST2 gene product, ST2LV. Biochim Biophys Acta. 2004; 1681:1–14.

3. Oboki K, Ohno T, Kajiwara N, Saito H, Nakae S. IL-33 and IL-33 receptors in host defense and diseases. Allergol Int. 2010; 59:143–160.

4. Trajkovic V, Sweet MJ, Xu D. T1/ST2--an IL-1 receptor-like modulator of immune responses. Cytokine Growth Factor Rev. 2004; 15:87–95.

5. Hayakawa H, Hayakawa M, Kume A, Tominaga S. Soluble ST2 blocks interleukin-33 signaling in allergic airway inflammation. J Biol Chem. 2007; 282:26369–26380.

6. Oshikawa K, Yanagisawa K, Tominaga S, Sugiyama Y. Expression and function of the ST2 gene in a murine model of allergic airway inflammation. Clin Exp Allergy. 2002; 32:1520–1526.

7. Ali M, Zhang G, Thomas WR, McLean CJ, Bizzintino JA, Laing IA, et al. Investigations into the role of ST2 in acute asthma in children. Tissue Antigens. 2009; 73:206–212.

8. Oshikawa K, Kuroiwa K, Tago K, Iwahana H, Yanagisawa K, Ohno S, et al. Elevated soluble ST2 protein levels in sera of patients with asthma with an acute exacerbation. Am J Respir Crit Care Med. 2001; 164:277–281.

9. Smith PH, Ownby DR. Clinical significance of immunoglobulin E. In : Adkinson NF, Busse WW, Bochner BS, Holgate ST, Simons FE, Lemanske RF, editors. Allergy: principles and practice. 7th ed. St Louis: Mosby;2008. p. 845–857.
10. Sweet MJ, Leung BP, Kang D, Sogaard M, Schulz K, Trajkovic V, et al. A novel pathway regulating lipopolysaccharide-induced shock by ST2/T1 via inhibition of Toll-like receptor 4 expression. J Immunol. 2001; 166:6633–6639.

11. Brunner M, Krenn C, Roth G, Moser B, Dworschak M, Jensen-Jarolim E, et al. Increased levels of soluble ST2 protein and IgG1 production in patients with sepsis and trauma. Intensive Care Med. 2004; 30:1468–1473.

12. Shimpo M, Morrow DA, Weinberg EO, Sabatine MS, Murphy SA, Antman EM, et al. Serum levels of the interleukin-1 receptor family member ST2 predict mortality and clinical outcome in acute myocardial infarction. Circulation. 2004; 109:2186–2190.

13. Yin H, Li XY, Liu T, Yuan BH, Zhang BB, Hu SL, et al. Adenovirus-mediated delivery of soluble ST2 attenuates ovalbumin-induced allergic asthma in mice. Clin Exp Immunol. 2012; 170:1–9.

14. Morrison PT, Thomas LH, Sharland M, Friedland JS. RSV-infected airway epithelial cells cause biphasic up-regulation of CCR1 expression on human monocytes. J Leukoc Biol. 2007; 81:1487–1495.

15. Chow JY, Wong CK, Cheung PF, Lam CW. Intracellular signaling mechanisms regulating the activation of human eosinophils by the novel Th2 cytokine IL-33: implications for allergic inflammation. Cell Mol Immunol. 2010; 7:26–34.

16. Pecaric-Petkovic T, Didichenko SA, Kaempfer S, Spiegl N, Dahinden CA. Human basophils and eosinophils are the direct target leukocytes of the novel IL-1 family member IL-33. Blood. 2009; 113:1526–1534.

17. Oboki K, Nakae S, Matsumoto K, Saito H. IL-33 and Airway Inflammation. Allergy Asthma Immunol Res. 2011; 3:81–88.

18. Kondo Y, Yoshimoto T, Yasuda K, Futatsugi-Yumikura S, Morimoto M, Hayashi N, et al. Administration of IL-33 induces airway hyperresponsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int Immunol. 2008; 20:791–800.

19. Préfontaine D, Nadigel J, Chouiali F, Audusseau S, Semlali A, Chakir J, et al. Increased IL-33 expression by epithelial cells in bronchial asthma. J Allergy Clin Immunol. 2010; 125:752–754.

20. Prefontaine D, Lajoie-Kadoch S, Foley S, Audusseau S, Olivenstein R, Halayko AJ, et al. Increased expression of IL-33 in severe asthma: evidence of expression by airway smooth muscle cells. J Immunol. 2009; 183:5094–5103.

21. Hamzaoui A, Berraies A, Kaabachi W, Haifa M, Ammar J, Kamel H. Induced sputum levels of IL-33 and soluble ST2 in young asthmatic children. J Asthma. 2013; 50:803–809.

22. Mazzarella G, Bianco A, Catena E, De Palma R, Abbate GF. Th1/Th2 lymphocyte polarization in asthma. Allergy. 2000; 55:Suppl 61. 6–9.

23. Teixeira LK, Fonseca BP, Barboza BA, Viola JP. The role of interferon-gamma on immune and allergic responses. Mem Inst Oswaldo Cruz. 2005; 100:Suppl 1. 137–144.

24. Corrigan CJ, Kay AB. CD4 T lymphocyte activation in acute severe asthma. Int Arch Allergy Appl Immunol. 1991; 94:270–271.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download