Abstract
Purpose
Little data is currently available on the use of the impulse oscillometry system (IOS) parameter in analyzing the lung function of young children with cough-variant asthma (CVA) and classic asthma. The aims of this study were to evaluate the bronchial responsiveness between patients with CVA and those with classic asthma using dose-response slope and various cutoff values.
Methods
A methacholine challenge test and a pulmonary function test were performed in 43 children with classic asthma and 26 children with CVA using IOS, and the respiratory resistance (Rrs) and reactance (Xrs) were obtained. The bronchial responsiveness were assessed by provocative concentration causing an 80% fall from baseline in reactance at 5 Hz (PC80_Xrs5) and a 40% increase in resistance at 5 Hz (PC40_Rrs5) and calculating from the degree of dose-response slope (DRS) for airway resistance and reactance.
Bronchial asthma is a main cause of chronic cough in children and cough-variant asthma (CVA), also called "hidden asthma," is diagnosed in children with chronic cough without other causative factors who show bronchial hyperresponsiveness and respond well to conventional asthma therapy.1) CVA is considered a precursor of classic asthma2) and 54% of the patients with CVA were found to develop classic asthma in the long-term follow-up studies.3,4) Moreover, irreversible pathological changes of the airways due to persistent inflammation may occur in both classic asthma and CVA.5) However little is known about the mechanism underlying CVA in children and how CVA differs from classic asthma.6)
The impulse oscillometry system (IOS), which requires only passive cooperation, has been extensively used in measuring the lung function of children with chronic cough.7) Since recent studies have suggested that IOS measurements have a higher sensitivity than forced expiratory volume in 1 second (FEV1) measured through spirometry in determining the obstruction of the peripheral airways,8,9) IOS has been more widely used in the diagnosis of asthma in children. However there are limitations in the practical application of the IOS parameters in CVA. Although there are some studies available on bronchial reactivity based on respiratory resistance (Rrs) in school aged children with CVA,10) the relationship between other IOS parameters including resistance and clinical expression of CVA in preschool children is still unclear. Therefore, we aimed to differentiate CVA from classic asthma on the basis of bronchial responsiveness using various IOS parameters in preschool children.
The participants were 26 children with CVA and 43 children with classic asthma who were referred to the Department of the Pediatric Allergy Clinic at the CHA Bundang Medical Center. The mean age of subjects was 5.3±2.3 years and all subjects tolerated the methacholine challenge testing (MCT) using IOS. We collected the data based on the "stringent index for the prediction of asthma."11) Past history of atopic eczema and allergic rhinitis of the patient and parental history of asthma diagnosed by doctors were considered as valid information.
The classic asthma group was recruited from subjects who had been previously diagnosed as asthma by medical doctors with recurrent episodes of wheeze and/or dyspnea. The patients had shown responses to intermittent use of inhaled β2-agonists and/or inhaled corticosteroids. The CVA was diagnosed with the presence of chronic cough over 8 weeks without dyspnea and wheeze, no discernible cause of the cough after clinical assessment and chest radiography, and bronchial hyperresponsiveness upon MCT, showing a clinical improvement with β2-agonist therapy.1,10,12)
We excluded the patients with current respiratory infection within one month prior to the study, such as chronic rhinosinusitis, gastroesophageal reflux, habitual cough, and bronchitis identified by physical examination and radiologic exam. Inhaled long acting or short acting β2-agonist and inhaled steroid therapy were withheld for at least 24-48 hours prior to the study. This study was approved by the Institutional Review Board at Bundang CHA Medical Center.
IOS was conducted using a Master Screen-IOS Digital Instrument (Jaeger Co., Würtzburg, Germany). Briefly, the real-time respiratory impedance was monitored. The breaths were considered useful for analysis only when a proper signal persisted for at least 20 seconds. After the baseline lung functions were measured twice with an interval of 10 minutes, MCT was performed.13) The mean Rrs and Xrs value was used as the baseline value. The oxygen saturation was continuously monitored during the testing.
MCT by IOS was performed in accordance with the American Thoracic Society (ATS) Standards14) using a Jaeger MasterScreen device (Jaeger, Würtzburg, Germany). Fresh solutions of methacholine were prepared by diluting methacholine in a phosphate buffer solution. We used a quadrupling concentration protocol (0.0625, 0.25, 1, 4, 16 mg/mL) in order to prevent subjects from being exhausted. The solutions were then delivered via a nebulizer (DeVilbiss Health Care Inc., Somerset, PA, USA) driven by a dosimeter (Pulmonary Data Service, Louisville, CO, USA) during the 5 inspiratory capacity breaths. The testing was stopped if the methacholine chloride dose reached the final concentration, oxygen saturation was below 91% or the patient complained of dyspnea, chest discomfort, or severe cough. The mean value of Rrs, Xrs, resonance frequency (Rf), and the area of reactance (AX) at 5, 10, 15, 20, 25, and 35 Hz at each methacholine dose were measured.
Previous study showed that more than a 40% increase in Rrs at 6 Hz (PC40_Rrs6) was valuable for diagnosing asthma15) and more than an 80% decrease from the baseline in Xrs at 5 Hz (PC80_Xrs5) was also considered as a significant marker to differentiate the asthmatics from controls.16) We assessed PC40_Rrs5 and PC80_Xrs5 using a modified equation described by the ATS guidelines for PC20_FEV117) and compared them between the two groups.
We also calculated dose-response slope (DRS) to measure the bronchial responsiveness. DRSs were obtained for Xrs and Rrs as the previous researchers proposed DRS for FEV1.18) First, we obtained the cumulative doses expressed in µmol instead of concentrations expressed in mg/mL in order to compare the results with other publications.19) In this study, quadrupling increments with the five-breath dosimeter method were used according to the ATS guideline.20) For the five-breath method, approximately 0.009 mL was delivered to the subject with each inhalation. The schedule was 5 inhalations of methacholine of 0.0625, 0.25, 1, 4, and 16 mg/mL (total cumulative dose 0.959 mg). The cumulative doses of methacholine converted into micromoles at each level were 0.014, 0.072, 0.302, 1.222, and 4.901 µmol, respectively, which were obtained using the molecular weight of methacholine chloride (195.7 g/mol). Hence, the concentrations used were 0.0625-16 mg/mL and the doses actually delivered were 0.014-4.901 µmol. The slope was defined as log (percent change in X/last cumulative dose), in which X represents Rrs and Xrs at various Hz because of their highly skewed distribution. The percent changes of parameters were calculated using the following formula: 100×(post value-baseline value)/(baseline value). Since some Xrs values ranged from negative to positive, we used absolute value of real numbers.
All analyses were performed with the SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA). All data were presented as mean±standard deviation, and P-values less than 0.05 were considered significant. Mann-Whitney U test was used when the two groups were to be compared. The results of DRS were transformed logarithmically because of skewed distribution.
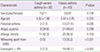
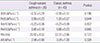
A summary of the demographic and clinical characteristics of participants are shown in Table 1. There were no statistical differences in age and sex between the two groups. The prevalence of parental asthma, wheezing without colds and eosinophil count greater than 4% were higher in classic asthma group than those in the CVA group (Table 1). The values of baseline lung function did not differ significantly between two groups (Table 2). For Xrs and Rrs, there were no statistical differences in the base pulmonary-function indices at various frequencies (15, 20, 25, and 35 Hz) between the two groups (data not shown).
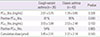
The bronchial hyperresponsiveness, sensitivity, concentration level and actual cumulative dose were compared (Table 3). PC80_Xrs5 and PC40_Rrs5 showed no significant differences between the two groups. However, positive PC80_Xrs5 (≤16 mg/mL) of the classic asthma group was significantly higher than that of the CVA group (P=0.040).
We also compare the values of DRS of resistance and reactance between the two groups. The mean value of DRS_Xrs5 in the classic asthma group was significantly steeper than that in the CVA group (1.70±0.46 vs. 1.48±0.43, P=0.040) (Fig. 1). There was no significant difference in the mean values of DRS_Rrs5 for the CVA subjects and the classic asthma subjects (1.13±0.41 vs. 1.23±0.46, P=0.342). In addition, there was no significant difference in DRS_Xrs10 and DRS_Rrs10 between the two groups (P=0.100 and P=0.206, respectively).0
In this study, we measured the bronchial hyperresponsiveness by various ways between the CVA subjects and the classic asthma subjects. We found that the value of two linear DRS of Xrs5 and positive PC80_Xrs5 were significantly higher in the classic asthma group than those in the CVA group.
Bronchial hyperresponsiveness is a characteristic of asthma and provocative concentration causing a 20% fall in FEV1 (PC20_FEV1) derived from spirometry and two-point linear DRS are often used as parameters to determine BHR.14,21,22) However, the conventional method is relatively hard to perform in young children and there have been previous studies comparing the conventional method and the IOS method; they suggested various cutoff points (such as PC40_Rrs6,15) PC70_Rrs12,23) and PC80_Xrs516) for diagnosing asthma as compatible for PC20_FEV1. In this study, we examined PC40_Rrs5 and PC80_Xrs5 in the classic asthma group and the CVA group. The provocative concentration was not significantly different between the two groups. However, the sensitivity of PC80_Xrs5 was significantly higher in the classic asthma group. Moreover, the two-point linear dose response slope, which is another gold standard determining BHR, was also calculated and the value of reactance at 5 Hz was higher in the classic asthma group than that in the CVA group. This result is comparable to the results by Mochizuki et al.10), who suggested another concept of Dmin (minimum dose of Methacholine) and SRs (slope of Methacholine dose-response curve) of resistance corresponding to provocative concentration and two linear DRS. They used 'unit' as the methacholine cumulative concentration; however, it is not actual cumulative dose of methacholine administered into the patient. Because the concentration of methacholine, method of inhalation, and output of nebulizer are different in previous studies, converting the concentration to actual dose is necessary to compare the results from different studies. Moreover, they only measured the DRS of resistance at 7 Hz. Therefore, it is difficult to compare the results between the study by Mochizuki et al.10) and our study directly. Despite the methodological differences between the two studies, the result that there was no statistical difference between Dmin in the CVA group and the classic asthma group is compatible to our result. They showed a significant difference in the dose-response curve of resistance at 7 Hz and sex between the two groups. On the contrary, we calculated the DRS of Rrs and Xrs at various Hz and showed that the DRS of reactance at 5 Hz was best for differentiating the classic asthma from CVA and there was no difference in sex.
Collectively, the reactance at 5 Hz is better than resistance at discriminating between the classic asthma and CVA on bronchial challenge testing using DRS. The mean value of the reactance at 5 Hz in patients with CVA was lower than that in patients with asthma, but there could be some overlaps in values of DRS_Xrs5 between two groups because of similar mechanism underlying CVA and classic asthma. Koh et al.24) suggested that the level of maximal airway response could be an important risk factor for the future development of classic asthma in patients with CVA, which implies that the CVA patients with higher airway response are more likely to develop classic asthma later in life and the CVA patients with lower airway response are less likely develop classic asthma.
There are some suggestions about the mechanism of lower bronchoconstriction in CVA subjects. The airway inflammation which is one of the common mechanisms underlying both CVA and asthma aggravates BHR and hypersensitivity of cough receptors.4,25) Furthermore, the airway remodeling occurred by inflammation might introduce the rigidity of airway walls in CVA subjects.10) Also, CVA is associated with a higher wheezing threshold and a relatively mild degree of maximal airway response at a similar degree of airway sensitivity to methacholine compared to classic asthma.26)
Reactance is a complex concept; it includes forces of inertia and the elastic recoil properties of the lung tissue. Also, it is correlated with peripheral heterogeneity, airway narrowing and airway wall shunting.27) Therefore, considering the rigidity of airway wall and airway wall shunting caused by widespread peripheral constriction which are related to lung elastance rather than airway caliber, the reactance may be better than resistance at demonstrating the characteristic of CVA.
In conclusion, the present study suggested that the use of DRS is useful for the differential diagnosis of classic asthma and CVA and it is best to measure the reactance at 5 Hz in preschool-aged children. More extensive research focusing on the assessment of asthma severity and the pathophysiology of both asthma and CVA is necessary to determine the clinical significance of Xrs5.
Figures and Tables
Fig. 1
Comparison of dose-response slope of reactance at 5 Hz (DRS_Xrs5) between the cough-variant asthma (CVA) group and the classic asthma group. The mean value of DRS_Xrs5 in the classic asthma group was significantly higher than that in the CVA group (P=0.04).

References
1. Irwin RS, French CT, Smyrnios NA, Curley FJ. Interpretation of positive results of a methacholine inhalation challenge and 1 week of inhaled bronchodilator use in diagnosing and treating cough-variant asthma. Arch Intern Med. 1997; 157:1981–1987.

3. Fujimura M, Ogawa H, Nishizawa Y, Nishi K. Comparison of atopic cough with cough variant asthma: is atopic cough a precursor of asthma? Thorax. 2003; 58:14–18.

4. Todokoro M, Mochizuki H, Tokuyama K, Morikawa A. Childhood cough variant asthma and its relationship to classic asthma. Ann Allergy Asthma Immunol. 2003; 90:652–659.

5. De Diego A, Martinez E, Perpina M, Nieto L, Compte L, Macian V, et al. Airway inflammation and cough sensitivity in cough-variant asthma. Allergy. 2005; 60:1407–1411.

6. Niimi A, Matsumoto H, Minakuchi M, Kitaichi M, Amitani R. Airway remodelling in cough-variant asthma. Lancet. 2000; 356:564–565.

7. Beydon N, Davis SD, Lombardi E, Allen JL, Arets HG, Aurora P, et al. An official American Thoracic Society/European Respiratory Society statement: pulmonary function testing in preschool children. Am J Respir Crit Care Med. 2007; 175:1304–1345.

8. Marotta A, Klinnert MD, Price MR, Larsen GL, Liu AH. Impulse oscillometry provides an effective measure of lung dysfunction in 4-year-old children at risk for persistent asthma. J Allergy Clin Immunol. 2003; 112:317–322.

9. Nielsen KG, Bisgaard H. Discriminative capacity of bronchodilator response measured with three different lung function techniques in asthmatic and healthy children aged 2 to 5 years. Am J Respir Crit Care Med. 2001; 164:554–559.

10. Mochizuki H, Arakawa H, Tokuyama K, Morikawa A. Bronchial sensitivity and bronchial reactivity in children with cough variant asthma. Chest. 2005; 128:2427–2434.

11. Castro-Rodriguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000; 162(4 Pt 1):1403–1406.

12. Niimi A, Amitani R, Suzuki K, Tanaka E, Murayama T, Kuze F. Eosinophilic inflammation in cough variant asthma. Eur Respir J. 1998; 11:1064–1069.

13. Oostveen E, MacLeod D, Lorino H, Farre R, Hantos Z, Desager K, et al. The forced oscillation technique in clinical practice: methodology, recommendations and future developments. Eur Respir J. 2003; 22:1026–1041.

14. Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, et al. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000; 161:309–329.
15. Broeders ME, Molema J, Hop WC, Folgering HT. Bronchial challenge, assessed with forced expiratory manoeuvres and airway impedance. Respir Med. 2005; 99:1046–1052.

16. Jee HM, Kwak JH, Jung DW, Han MY. Useful parameters of bronchial hyperresponsiveness measured with an impulse oscillation technique in preschool children. J Asthma. 2010; 47:227–232.

17. Statement of the American Thoracic Society. Standardization of spirometry: 1987 update. Am Rev Respir Dis. 1987; 136:1285–1298.
18. Izbicki G, Bar-Yishay E. Methacholine inhalation challenge: a shorter, cheaper and safe approach. Eur Respir J. 2001; 17:46–51.

19. Ownby DR, Peterson EL, Johnson CC. Factors related to methacholine airway responsiveness in children. Am J Respir Crit Care Med. 2000; 161:1578–1583.

20. Jayet PY, Schindler C, Kunzli N, Zellweger JP, Brandli O, Perruchoud AP, et al. Reference values for methacholine reactivity (SAPALDIA study). Respir Res. 2005; 6:131.

21. Bohadana AB, Peslin R, Megherbi SE, Teculescu D, Sauleau EA, Wild P, et al. Dose-response slope of forced oscillation and forced expiratory parameters in bronchial challenge testing. Eur Respir J. 1999; 13:295–300.

22. Garcia-Rio F, Mediano O, Ramirez M, Vinas A, Alonso A, Alvarez-Sala R, et al. Usefulness of bronchial reactivity analysis in the diagnosis of bronchial asthma in patients with bronchial hyperresponsiveness. Respir Med. 2004; 98:199–204.

23. Bouaziz N, Beyaert C, Gauthier R, Monin P, Peslin R, Marchal F. Respiratory system reactance as an indicator of the intrathoracic airway response to methacholine in children. Pediatr Pulmonol. 1996; 22:7–13.

24. Koh YY, Park Y, Kim CK. The importance of maximal airway response to methacholine in the prediction of wheezing development in patients with cough-variant asthma. Allergy. 2002; 57:1165–1170.

25. Koh YY, Jeong JH, Park Y, Kim CK. Development of wheezing in patients with cough variant asthma during an increase in airway responsiveness. Eur Respir J. 1999; 14:302–308.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download