This article has been corrected. See "Erratum: Reference Correction. Updates on desensitization for hypersensitivity reactions related to chemotherapy" in Volume 2 on page 150.
Abstract
As the use of chemotherapeutic agents increased rapidly in recent years, more patients are under the potential risk of chemotherapy related adverse reactions. Multiple regular exposures to the same drug by chemotherapy protocol may increase the risk of sensitization to a chemotherapeutic agent, which can result in hypersensitivity reactions. Once severe hypersensitivity reactions occur, causative drugs should be avoided. However, a substitute with equal efficacy is not always available. When there is no effective alternative, desensitization is a safe tool for maintenance of chemotherapeutic agents causing hypersensitivity reaction. In this review, we introduce the latest knowledge about desensitization protocol for chemotherapeutic agents which are frequently used recently.
Figures and Tables
Table 1
Incidence and severity of hypersensitivity to platinum agents

Reproduced from Makrilia et al. Met Based Drugs 2010;2010. Article ID 207084.54)
Table 2
An example of 12-step desensitization protocol
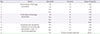
Reproduced from Makrilia et al. Met Based Drugs 2010;2010. Article ID 207084.54)
Table 3
Premedication protocol for paclitaxel hypersensitivity used in Cleveland Clinic Cancer Center25)

References
2. Callahan MB, Lachance JA, Stone RL, Kelsey J, Rice LW, Jazaeri AA. Use of cisplatin without desensitization after carboplatin hypersensitivity reaction in epithelial ovarian and primary peritoneal cancer. Am J Obstet Gynecol. 2007; 197:199.e1–199.e4.

3. Smith IE, Harland SJ, Robinson BA, Evans BD, Goodhart LC, Calvert AH, et al. Carboplatin: a very active new cisplatin analog in the treatment of small cell lung cancer. Cancer Treat Rep. 1985; 69:43–46.
4. Rothenberg ML, Oza AM, Bigelow RH, Berlin JD, Marshall JL, Ramanathan RK, et al. Superiority of oxaliplatin and fluorouracil-leucovorin compared with either therapy alone in patients with progressive colorectal cancer after irinotecan and fluorouracil-leucovorin: interim results of a phase III trial. J Clin Oncol. 2003; 21:2059–2069.

5. Markman M, Kennedy A, Webster K, Elson P, Peterson G, Kulp B, et al. Clinical features of hypersensitivity reactions to carboplatin. J Clin Oncol. 1999; 17:1141.

6. Siu SW, Chan RT, Au GK. Hypersensitivity reactions to oxaliplatin: experience in a single institute. Ann Oncol. 2006; 17:259–261.

7. Syrigou E, Syrigos K, Saif MW. Hypersensitivity reactions to oxaliplatin and other antineoplastic agents. Curr Allergy Asthma Rep. 2008; 8:56–62.

8. Lee C, Gianos M, Klaustermeyer WB. Diagnosis and management of hypersensitivity reactions related to common cancer chemotherapy agents. Ann Allergy Asthma Immunol. 2009; 102:179–187.

9. Pagani M. The complex clinical picture of presumably allergic side effects to cytostatic drugs: symptoms, pathomechanism, reexposure, and desensitization. Med Clin North Am. 2010; 94:835–852.

10. Lee CW, Matulonis UA, Castells MC. Carboplatin hypersensitivity: a 6-h 12-step protocol effective in 35 desensitizations in patients with gynecological malignancies and mast cell/IgE-mediated reactions. Gynecol Oncol. 2004; 95:370–376.

11. Lee CW, Matulonis UA, Castells MC. Rapid inpatient/outpatient desensitization for chemotherapy hypersensitivity: standard protocol effective in 57 patients for 255 courses. Gynecol Oncol. 2005; 99:393–399.

12. Cortijo-Cascajares S, Nacle-Lopez I, Garcia-Escobar I, Aguilella-Vizcaino MJ, Herreros-de-Tejada A, Cortes-Funes Castro H, et al. Effectiveness of oxaliplatin desensitization protocols. Clin Transl Oncol. 2013; 15:219–225.

13. Garufi C, Vaglio S, Brienza S, Conti L, D'Attino RM, Girelli G, et al. Immunohemolytic anemia following oxaliplatin administration. Ann Oncol. 2000; 11:497.

14. Ichikawa Y, Goto A, Hirokawa S, Kijima M, Ishikawa T, Chishima T, et al. Allergic reactions to oxaliplatin in a single institute in Japan. Jpn J Clin Oncol. 2009; 39:616–620.

15. Castells MC, Tennant NM, Sloane DE, Hsu FI, Barrett NA, Hong DI, et al. Hypersensitivity reactions to chemotherapy: outcomes and safety of rapid desensitization in 413 cases. J Allergy Clin Immunol. 2008; 122:574–580.

16. Shaw JL, Fakhri S, Citardi MJ, Porter PC, Corry DB, Kheradmand F, et al. IL-33-responsive innate lymphoid cells are an important source of IL-13 in chronic rhinosinusitis with nasal polyps. Am J Respir Crit Care Med. 2013; 188:432–439.

17. Meyer L, Zuberbier T, Worm M, Oettle H, Riess H. Hypersensitivity reactions to oxaliplatin: cross-reactivity to carboplatin and the introduction of a desensitization schedule. J Clin Oncol. 2002; 20:1146–1147.

18. Gammon D, Bhargava P, McCormick MJ. Hypersensitivity reactions to oxaliplatin and the application of a desensitization protocol. Oncologist. 2004; 9:546–549.

19. Mis L, Fernando NH, Hurwitz HI, Morse MA. Successful desensitization to oxaliplatin. Ann Pharmacother. 2005; 39:966–969.

20. Nozawa H, Muto Y, Yamada Y. Desensitization to oxaliplatin with two stages of premedication in a patient with metastatic rectal cancer. Clin Ther. 2008; 30:1160–1165.

21. Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1971; 93:2325–2327.

22. Yared JA, Tkaczuk KH. Update on taxane development: new analogs and new formulations. Drug Des Devel Ther. 2012; 6:371–384.
24. Rowinsky EK, Eisenhauer EA, Chaudhry V, Arbuck SG, Donehower RC. Clinical toxicities encountered with paclitaxel (Taxol). Semin Oncol. 1993; 20:4 Suppl 3. 1–15.
25. Markman M, Kennedy A, Webster K, Peterson G, Kulp B, Belinson J. An effective and more convenient drug regimen for prophylaxis against paclitaxel-associated hypersensitivity reactions. J Cancer Res Clin Oncol. 1999; 125:427–429.

26. Tankanow RM. Docetaxel: a taxoid for the treatment of metastatic breast cancer. Am J Health Syst Pharm. 1998; 55:1777–1791.

27. Markman M, Kennedy A, Webster K, Kulp B, Peterson G, Belinson J. Paclitaxel-associated hypersensitivity reactions: experience of the gynecologic oncology program of the Cleveland Clinic Cancer Center. J Clin Oncol. 2000; 18:102–105.

28. Peereboom DM, Donehower RC, Eisenhauer EA, McGuire WP, Onetto N, Hubbard JL, et al. Successful re-treatment with taxol after major hypersensitivity reactions. J Clin Oncol. 1993; 11:885–890.

29. Berger MJ, Dunlea LJ, Rettig AE, Lustberg MB, Phillips GS, Shapiro CL. Feasibility of stopping paclitaxel premedication after two doses in patients not experiencing a previous infusion hypersensitivity reaction. Support Care Cancer. 2012; 20:1991–1997.

30. Schwartz JR. Dexamethasone premedication for prophylaxis of taxane toxicities: can the doses be reduced when paclitaxel or docetaxel are given weekly? J Oncol Pharm Pract. 2012; 18:250–256.

31. Feldweg AM, Lee CW, Matulonis UA, Castells M. Rapid desensitization for hypersensitivity reactions to paclitaxel and docetaxel: a new standard protocol used in 77 successful treatments. Gynecol Oncol. 2005; 96:824–829.

32. Joerger M. Prevention and handling of acute allergic and infusion reactions in oncology. Ann Oncol. 2012; 23:Suppl 10. x313–x319.

33. Gelderblom H, Verweij J, Nooter K, Sparreboom A. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001; 37:1590–1598.
34. Szebeni J, Alving CR, Savay S, Barenholz Y, Priev A, Danino D, et al. Formation of complement-activating particles in aqueous solutions of Taxol: possible role in hypersensitivity reactions. Int Immunopharmacol. 2001; 1:721–735.

35. Szebeni J, Muggia FM, Alving CR. Complement activation by Cremophor EL as a possible contributor to hypersensitivity to paclitaxel: an in vitro study. J Natl Cancer Inst. 1998; 90:300–306.

36. Weiszhár Z, Czúcz J, Révész C, Rosivall L, Szebeni J, Rozsnyay Z. Complement activation by polyethoxylated pharmaceutical surfactants: Cremophor-EL, Tween-80 and Tween-20. Eur J Pharm Sci. 2012; 45:492–498.

37. Decorti G, Bartoli Klugmann F, Candussio L, Baldini L. Effect of paclitaxel and Cremophor EL on mast cell histamine secretion and their interaction with adriamycin. Anticancer Res. 1996; 16:317–320.
38. Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005; 23:7794–7803.

39. Essayan DM, Kagey-Sobotka A, Colarusso PJ, Lichtenstein LM, Ozols RF, King ED. Successful parenteral desensitization to paclitaxel. J Allergy Clin Immunol. 1996; 97(1 Pt 1):42–46.

40. Lokich J, Anderson N. Paclitaxel hypersensitivity reactions: a role for docetaxel substitution. Ann Oncol. 1998; 9:573.

41. Denman JP, Gilbar PJ, Abdi EA. Hypersensitivity reaction (HSR) to docetaxel after a previous HSR to paclitaxel. J Clin Oncol. 2002; 20:2760–2761.

42. Fishman A, Gold T, Goldberg A, Confino-Cohen R, Beyth Y, Menczer J, et al. Effective desensitization protocol to paclitaxel following hypersensitivity reaction. Int J Gynecol Cancer. 1999; 9:156–159.

43. Syrigou E, Dannos I, Kotteas E, Makrilia N, Tourkantonis I, Dilana K, et al. Hypersensitivity reactions to docetaxel: retrospective evaluation and development of a desensitization protocol. Int Arch Allergy Immunol. 2011; 156:320–324.

44. Gastaminza G, de la Borbolla JM, Goikoetxea MJ, Escudero R, Anton J, Espinos J, et al. A new rapid desensitization protocol for chemotherapy agents. J Investig Allergol Clin Immunol. 2011; 21:108–112.
45. Sacks PL, Snidvongs K, Rom D, Earls P, Sacks R, Harvey RJ. The impact of neo-osteogenesis on disease control in chronic rhinosinusitis after primary surgery. Int Forum Allergy Rhinol. 2013; 3:823–827.

46. Syrigou E, Makrilia N, Koti I, Saif MW, Syrigos KN. Hypersensitivity reactions to antineoplastic agents: an overview. Anticancer Drugs. 2009; 20:1–6.

47. Bonno M, Kawasaki H, Hori H, Umemoto M, Komada Y, Sakurai M. Rapid desensitization for L-asparaginase hypersensitivity. J Allergy Clin Immunol. 1998; 101(4 Pt 1):571–572.

48. Vrooman LM, Supko JG, Neuberg DS, Asselin BL, Athale UH, Clavell L, et al. Erwinia asparaginase after allergy to E. coli asparaginase in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2010; 54:199–205.

49. Akbayram S, Dogan M, Akgun C, Caksen H, Oner AF. A desensitization protocol in children with L-asparaginase hypersensitivity. J Pediatr Hematol Oncol. 2010; 32:e187–e191.

50. Farnam K, Chang C, Teuber S, Gershwin ME. Nonallergic drug hypersensitivity reactions. Int Arch Allergy Immunol. 2012; 159:327–345.

51. Kidera Y, Satoh T, Ueda S, Okamoto W, Okamoto I, Fumita S, et al. High-dose dexamethasone plus antihistamine prevents colorectal cancer patients treated with modified FOLFOX6 from hypersensitivity reactions induced by oxaliplatin. Int J Clin Oncol. 2011; 16:244–249.

52. Kim MY, Kang SY, Lee SY, Yang MS, Kim MH, Song WJ, et al. Hypersensitivity reactions to oxaliplatin: clinical features and risk factors in Koreans. Asian Pac J Cancer Prev. 2012; 13:1209–1215.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download