Abstract
Purpose
Topical calcineurin inhibitor is recently developed topical immunomodulator, and preliminary studies showed its effectiveness in the treatment of atopic dermatitis (AD). However, some side effects including transient irritation can influence the patient compliance. So, there are some needs to improve the patient compliance. The purpose of this study was to evaluate the efficacy, safety and patient compliance with using topical tacrolimus 0.1% to treat AD when the correct information about topical tacrolimus are properly given to patients.
Methods
We examined the medical recordings, clinical severity scoring of total 194 AD patients at 9 general hospitals in Seoul, Korea from September 2010 to August 2011. We offered an infosheet of topical tacrolimus 0.1% and the patients applied it twice a day for 2 weeks. And we measured the efficacy of the topical tacrolimus 0.1% with SCORing atopic dermatitis (SCORAD) index, patient's global assessment (PGA), and investigator's global assessment (IGA).
Results
Topical tacrolimus 0.1% effectively controlled AD with a reduction of the SCORAD index from baseline 31.9 to 20.2 at 2 weeks of application. In IGA results showed 98% got improvement and in PGA, results showed 96% got improvement after treatment. Although 42.3% of the patients complained of adverse effects, these were all transient. The effect of information on topical tacrolimus 0.1% showed 34% patients could predict the side effect, 35% patients could feel safety to use, and 18% patients experienced side effect but could maintain topical calcineurin inhibitor.
Figures and Tables
 | Fig. 1Information about 0.1% tacrolimus includes method of applying and expected side effect such as irritation. |
 | Fig. 2SCORing atopic dermatitis (SCORAD) score change after treatment. All the values including SCORAD, A score (extent), B score (intensity), C score (subjective symptoms, pruritus+sleep loss) decreased significantly after treatment (*P<0.05). |
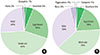 | Fig. 3(A) Investigator's global assessment and (B) patient's global assessment. Both shows similar improvement. |
References
1. Kabashima K. New concept of the pathogenesis of atopic dermatitis: interplay among the barrier, allergy, and pruritus as a trinity. J Dermatol Sci. 2013; 70:3–11.

4. Chase EP, Armstrong AW. Advances in management of atopic dermatitis: new therapies and novel uses of existing treatments. Semin Cutan Med Surg. 2012; 31:17–24.

6. Gupta AK, Adamiak A, Chow M. Tacrolimus: a review of its use for the management of dermatoses. J Eur Acad Dermatol Venereol. 2002; 16:100–114.

7. Katoh N, Hirano S, Yasuno H, Kishimoto S. Effects of tacrolimus ointment on facial eruption, itch, and scratching in patients with atopic dermatitis. J Dermatol. 2004; 31:194–199.

8. Hanifin JM, Ling MR, Langley R, Breneman D, Rafal E. Tacrolimus ointment for the treatment of atopic dermatitis in adult patients: part I, efficacy. J Am Acad Dermatol. 2001; 44:1 Suppl. S28–S38.

9. Soter NA, Fleischer AB Jr, Webster GF, Monroe E, Lawrence I. Tacrolimus ointment for the treatment of atopic dermatitis in adult patients: part II, safety. J Am Acad Dermatol. 2001; 44:1 Suppl. S39–S46.

10. Kapp A, Allen BR, Reitamo S. Atopic dermatitis management with tacrolimus ointment (Protopic). J Dermatolog Treat. 2003; 14:Suppl 1. 5–16.

11. Ruzicka T, Bieber T, Schopf E, Rubins A, Dobozy A, Bos JD, et al. European Tacrolimus Multicenter Atopic Dermatitis Study Group. A short-term trial of tacrolimus ointment for atopic dermatitis. N Engl J Med. 1997; 337:816–821.

12. Won CH, Seo PG, Park YM, Yang JM, Lee KH, Sung KJ, et al. A multicenter trial of the efficacy and safety of 0.03% tacrolimus ointment for atopic dermatitis in Korea. J Dermatolog Treat. 2004; 15:30–34.

13. Choi WW, Seo PG, Kim KH. Tacrolimus ointment; an open study for effects on severe facial atopic dermatitis in Korean. Ann Dermatol. 2002; 14:195–199.

14. Kim HO, Park CW, Lee CH, Lee JO. Efficacy of 0.1% Tacrolimus ment in Korean patients with atopic dermatitisoint. Korean J Dermatol. 2005; 43:312–318.
15. Luoma R, Koivikko A, Viander M. Development of asthma, allergic rhinitis and atopic dermatitis by the age of five years: a prospective study of 543 newborns. Allergy. 1983; 38:339–346.

16. Park YM, Park HJ, Kim TY, Kim CW. The study on the hospital - based relative frequency, and clinical and laboratory findings of atopic dermatitis. Korean J Dermatol. 1997; 35:96–106.
17. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh). 1980; Suppl 92. 44–47.
18. Williams HC. On the definition and epidemiology of atopic dermatitis. Dermatol Clin. 1995; 13:649–657.

19. Lee HJ, Cho SH, Ha SJ, Ahn WK, Park YM, Byun DG, et al. Minor cutaneous features of atopic dermatitis in South Korea. Int J Dermatol. 2000; 39:337–342.

20. Kim BJ, Kim MN, Kim KH, Kim DW, Ro YS, Park CW, et al. Multicenter survey of the efficacy and compliance with usingtopical pimecrolimus by patients with atopic dermatitis. Korean J Dermatol. 2008; 46:1357–1361.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download