Abstract
Atopic dermatitis is a chronic relapsing eczematous dermatosis, which usually starts in childhood, and various causes are intricately associated with the development of the disease. Recently, various abnormalities in barrier function have been identified as the cause of atopic dermatitis. Loss-of-function mutation of filaggrin, a significant constituent of skin barrier, has been revealed as a cause for atopic dermatitis, and factors like enhanced protease activity, and decreased synthesis of the lipid lamellae especially ceramides also plays an important role in barrier dysfunction. Not only these genetic causes but also environmental factors are associated in barrier dysfunction, such as soap or detergents which increases skin pH, or proteases of dust mites or cockroaches which enhances epidermal barrier breakdown. Lately, skin barrier dysfunction is also thought to play an important role in the early stage of other allergic diseases such as asthma. Therefore, comprehension of the function of skin barrier can provide help in understanding various allergic diseases.
Figures and Tables
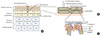 | Fig. 1Normal skin anatomy. (A) Normal skin is composed of five distinct layers: stratum basale, stratum spinosum, stratum granulosum, and stratum corneum. Among these five parts, the uppermost layer-stratum corneum- is responsible for skin barrier's function and supplements the functions of tight junction in the granular layer. (B) The brick and mortar model. When the granular layer is observed closely, it is composed of stacked up bricks of corneocytes made up of proteins and lipids surrounded by lipid-renriched intercellular matrix like a mortar. (C) The cornified envelope. The protein layer of the cornified envelope is composed of proteins such as involucrin, loricrin, and small proline-rich (SPR). Filaggrin, which binds keratin fibers, also plays an important role in the skin barrier function. The lipid layer of corneocyte is usually composed of lipid substances that are attached to involucrin. |
References
2. Williams HC. Williams HC, editor. What is atopic dermatitis and how should it be defined in epidemiological studies? Atopic dermatitis: the epidemiology, causes, and prevention of atopic eczema. 2000. New York: Cambridge University Press;3–24.

3. Elias PM, Wood LC, Feingold KR. Epidermal pathogenesis of inflammatory dermatoses. Am J Contact Dermat. 1999. 10:119–126.

4. Taïeb A. Hypothesis: from epidermal barrier dysfunction to atopic disorders. Contact Dermatitis. 1999. 41:177–180.

5. Lee SH, Jeong SK, Ahn SK. An update of the defensive barrier function of skin. Yonsei Med J. 2006. 47:293–306.

6. De Benedetto A, Rafaels NM, McGirt LY, Ivanov AI, Georas SN, Cheadle C, et al. Tight junction defects in patients with atopic dermatitis. J Allergy Clin Immunol. 2011. 127:773–786.e1-7.

7. De Benedetto A, Slifka MK, Rafaels NM, Kuo IH, Georas SN, Boguniewicz M, et al. Reductions in claudin-1 may enhance susceptibility to herpes simplex virus 1 infections in atopic dermatitis. J Allergy Clin Immunol. 2011. 128:242–246.e5.

8. Kubo A, Nagao K, Amagai M. Epidermal barrier dysfunction and cutaneous sensitization in atopic diseases. J Clin Invest. 2012. 122:440–447.

9. Elias PM. Epidermal lipids, barrier function, and desquamation. J Invest Dermatol. 1983. 80:1 Suppl. 44S–49S.

10. Serre G, Mils V, Haftek M, Vincent C, Croute F, Reano A, et al. Identification of late differentiation antigens of human cornified epithelia, expressed in re-organized desmosomes and bound to cross-linked envelope. J Invest Dermatol. 1991. 97:1061–1072.

11. Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol. 2005. 6:328–340.

12. Steinert PM, Marekov LN. The proteins elafin, filaggrin, keratin intermediate filaments, loricrin, and small proline-rich proteins 1 and 2 are isodipeptide cross-linked components of the human epidermal cornified cell envelope. J Biol Chem. 1995. 270:17702–17711.

13. Steven AC, Steinert PM. Protein composition of cornified cell envelopes of epidermal keratinocytes. J Cell Sci. 1994. 107(Pt 2):693–700.

14. Elias PM, Menon GK. Structural and lipid biochemical correlates of the epidermal permeability barrier. Adv Lipid Res. 1991. 24:1–26.

15. Steinert PM, Cantieri JS, Teller DC, Lonsdale-Eccles JD, Dale BA. Characterization of a class of cationic proteins that specifically interact with intermediate filaments. Proc Natl Acad Sci U S A. 1981. 78:4097–4101.

16. Harding CR, Watkinson A, Rawlings AV, Scott IR. Dry skin, moisturization and corneodesmolysis. Int J Cosmet Sci. 2000. 22:21–52.

17. Lavker RM. Membrane coating granules: the fate of the discharged lamellae. J Ultrastruct Res. 1976. 55:79–86.

18. Rawlings AV. Trends in stratum corneum research and the management of dry skin conditions. Int J Cosmet Sci. 2003. 25:63–95.

19. Hara J, Higuchi K, Okamoto R, Kawashima M, Imokawa G. High-expression of sphingomyelin deacylase is an important determinant of ceramide deficiency leading to barrier disruption in atopic dermatitis. J Invest Dermatol. 2000. 115:406–413.

20. Elias PM, Ghadially R. The aged epidermal permeability barrier: basis for functional abnormalities. Clin Geriatr Med. 2002. 18:103–120. vii
21. Fluhr JW, Kao J, Jain M, Ahn SK, Feingold KR, Elias PM. Generation of free fatty acids from phospholipids regulates stratum corneum acidification and integrity. J Invest Dermatol. 2001. 117:44–51.

22. De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol. 2012. 132(3 Pt 2):949–963.

23. O'Neill CA, Garrod D. Tight junction proteins and the epidermis. Exp Dermatol. 2011. 20:88–91.
24. Pummi K, Malminen M, Aho H, Karvonen SL, Peltonen J, Peltonen S. Epidermal tight junctions: ZO-1 and occludin are expressed in mature, developing, and affected skin and in vitro differentiating keratinocytes. J Invest Dermatol. 2001. 117:1050–1058.

25. Ishida-Yamamoto A, Kishibe M, Murakami M, Honma M, Takahashi H, Iizuka H. Lamellar granule secretion starts before the establishment of tight junction barrier for paracellular tracers in mammalian epidermis. PLoS One. 2012. 7:e31641.

27. Metz-Boutigue MH, Shooshtarizadeh P, Prevost G, Haikel Y, Chich JF. Antimicrobial peptides present in mammalian skin and gut are multifunctional defence molecules. Curr Pharm Des. 2010. 16:1024–1039.

28. Braff MH, Di Nardo A, Gallo RL. Keratinocytes store the antimicrobial peptide cathelicidin in lamellar bodies. J Invest Dermatol. 2005. 124:394–400.

29. Kim BE, Leung DY. Epidermal barrier in atopic dermatitis. Allergy Asthma Immunol Res. 2012. 4:12–16.

30. Wolf R, Wolf D. Abnormal epidermal barrier in the pathogenesis of atopic dermatitis. Clin Dermatol. 2012. 30:329–334.

31. Hogan MB, Peele K, Wilson NW. Skin barrier function and its importance at the start of the atopic march. J Allergy (Cairo). 2012. 2012:901940.

32. Knor T, Meholjic-Fetahovic A, Mehmedagic A. Stratum corneum hydration and skin surface pH in patients with atopic dermatitis. Acta Dermatovenerol Croat. 2011. 19:242–247.
33. Addor FA, Takaoka R, Rivitti EA, Aoki V. Atopic dermatitis: correlation between non-damaged skin barrier function and disease activity. Int J Dermatol. 2012. 51:672–676.

34. Irvine AD, McLean WH. Breaking the (un)sound barrier: filaggrin is a major gene for atopic dermatitis. J Invest Dermatol. 2006. 126:1200–1202.

35. Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006. 38:441–446.

36. O'Regan GM, Irvine AD. The role of filaggrin in the atopic diathesis. Clin Exp Allergy. 2010. 40:965–972.
37. Barnes KC. An update on the genetics of atopic dermatitis: scratching the surface in 2009. J Allergy Clin Immunol. 2010. 125:16–29.e1-11.

38. Smith FJ, Irvine AD, Terron-Kwiatkowski A, Sandilands A, Campbell LE, Zhao Y, et al. Loss-of-function mutations in the gene encoding filaggrin cause ichthyosis vulgaris. Nat Genet. 2006. 38:337–342.

39. Heimall J, Spergel JM. Filaggrin mutations and atopy: consequences for future therapeutics. Expert Rev Clin Immunol. 2012. 8:189–197.

40. Cascella R, Foti Cuzzola V, Lepre T, Galli E, Moschese V, Chini L, et al. Full sequencing of the FLG gene in Italian patients with atopic eczema: evidence of new mutations, but lack of an association. J Invest Dermatol. 2011. 131:982–984.

41. Winge MC, Bilcha KD, Lieden A, Shibeshi D, Sandilands A, Wahlgren CF, et al. Novel filaggrin mutation but no other loss-of-function variants found in Ethiopian patients with atopic dermatitis. Br J Dermatol. 2011. 165:1074–1080.

42. Nemoto-Hasebe I, Akiyama M, Nomura T, Sandilands A, McLean WH, Shimizu H. FLG mutation p.Lys4021X in the C-terminal imperfect filaggrin repeat in Japanese patients with atopic eczema. Br J Dermatol. 2009. 161:1387–1390.

43. Chen H, Ho JC, Sandilands A, Chan YC, Giam YC, Evans AT, et al. Unique and recurrent mutations in the filaggrin gene in Singaporean Chinese patients with ichthyosis vulgaris. J Invest Dermatol. 2008. 128:1669–1675.

44. Tang HY, Tang XF, Zuo XB, Gao JP, Sheng YJ, Li Y, et al. Association analysis of single nucleotide polymorphisms at five loci: comparison between atopic dermatitis and asthma in the Chinese Han population. PLoS One. 2012. 7:e35334.

45. Brown SJ, McLean WH. One remarkable molecule: filaggrin. J Invest Dermatol. 2012. 132(3 Pt 2):751–762.

46. Weidinger S, Illig T, Baurecht H, Irvine AD, Rodriguez E, Diaz-Lacava A, et al. Loss-of-function variations within the filaggrin gene predispose for atopic dermatitis with allergic sensitizations. J Allergy Clin Immunol. 2006. 118:214–219.

47. Howell MD, Kim BE, Gao P, Grant AV, Boguniewicz M, Debenedetto A, et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol. 2007. 120:150–155.

48. Scharschmidt TC, Man MQ, Hatano Y, Crumrine D, Gunathilake R, Sundberg JP, et al. Filaggrin deficiency confers a paracellular barrier abnormality that reduces inflammatory thresholds to irritants and haptens. J Allergy Clin Immunol. 2009. 124:496–506.

49. Oyoshi MK, Murphy GF, Geha RS. Filaggrin-deficient mice exhibit TH17-dominated skin inflammation and permissiveness to epicutaneous sensitization with protein antigen. J Allergy Clin Immunol. 2009. 124:485–493.

50. Moniaga CS, Egawa G, Kawasaki H, Hara-Chikuma M, Honda T, Tanizaki H, et al. Flaky tail mouse denotes human atopic dermatitis in the steady state and by topical application with Dermatophagoides pteronyssinus extract. Am J Pathol. 2010. 176:2385–2393.

51. de Guzman Strong C, Conlan S, Deming CB, Cheng J, Sears KE, Segre JA. A milieu of regulatory elements in the epidermal differentiation complex syntenic block: implications for atopic dermatitis and psoriasis. Hum Mol Genet. 2010. 19:1453–1460.

52. Esparza-Gordillo J, Weidinger S, Folster-Holst R, Bauerfeind A, Ruschendorf F, Patone G, et al. A common variant on chromosome 11q13 is associated with atopic dermatitis. Nat Genet. 2009. 41:596–601.

53. Henry J, Hsu CY, Haftek M, Nachat R, de Koning HD, Gardinal-Galera I, et al. Hornerin is a component of the epidermal cornified cell envelopes. FASEB J. 2011. 25:1567–1576.

54. Pellerin L, Henry J, Hsu CY, Balica S, Jean-Decoster C, Mechin MC, et al. Defects of filaggrin-like proteins in both lesional and nonlesional atopic skin. J Allergy Clin Immunol. 2013. 02. 09. [Epub]. http://dx.doi.org/10.1016/j.jaci.2012.12.1566.

55. Hansmann B, Ahrens K, Wu Z, Proksch E, Meyer-Hoffert U, Schroder JM. Murine filaggrin-2 is involved in epithelial barrier function and down-regulated in metabolically induced skin barrier dysfunction. Exp Dermatol. 2012. 21:271–276.

56. Wu Z, Hansmann B, Meyer-Hoffert U, Glaser R, Schrader JM. Molecular identification and expression analysis of filaggrin-2, a member of the S100 fused-type protein family. PLoS One. 2009. 4:e5227.

57. Broccardo CJ, Mahaffey S, Schwarz J, Wruck L, David G, Schlievert PM, et al. Comparative proteomic profiling of patients with atopic dermatitis based on history of eczema herpeticum infection and Staphylococcus aureus colonization. J Allergy Clin Immunol. 2011. 127:186–193.

58. Imokawa G, Abe A, Jin K, Higaki Y, Kawashima M, Hidano A. Decreased level of ceramides in stratum corneum of atopic dermatitis: an etiologic factor in atopic dry skin? J Invest Dermatol. 1991. 96:523–526.

59. Choi MJ, Maibach HI. Role of ceramides in barrier function of healthy and diseased skin. Am J Clin Dermatol. 2005. 6:215–223.

60. Murata Y, Ogata J, Higaki Y, Kawashima M, Yada Y, Higuchi K, et al. Abnormal expression of sphingomyelin acylase in atopic dermatitis: an etiologic factor for ceramide deficiency? J Invest Dermatol. 1996. 106:1242–1249.

61. Proksch E, Brandner JM, Jensen JM. The skin: an indispensable barrier. Exp Dermatol. 2008. 17:1063–1072.

62. Baroni A, Buommino E, De Gregorio V, Ruocco E, Ruocco V, Wolf R. Structure and function of the epidermis related to barrier properties. Clin Dermatol. 2012. 30:257–262.

63. Behne MJ, Meyer JW, Hanson KM, Barry NP, Murata S, Crumrine D, et al. NHE1 regulates the stratum corneum permeability barrier homeostasis. Microenvironment acidification assessed with fluorescence lifetime imaging. J Biol Chem. 2002. 277:47399–47406.
64. Krien PM, Kermici M. Evidence for the existence of a self-regulated enzymatic process within the human stratum corneum -an unexpected role for urocanic acid. J Invest Dermatol. 2000. 115:414–420.

65. Houben E, Hachem JP, De Paepe K, Rogiers V. Epidermal ceramidase activity regulates epidermal desquamation via stratum corneum acidification. Skin Pharmacol Physiol. 2008. 21:111–118.

66. Rawlings AV, Voegeli R. Stratum corneum proteases and dry skin conditions. Cell Tissue Res. 2013. 351:217–235.

67. Hachem JP, Man MQ, Crumrine D, Uchida Y, Brown BE, Rogiers V, et al. Sustained serine proteases activity by prolonged increase in pH leads to degradation of lipid processing enzymes and profound alterations of barrier function and stratum corneum integrity. J Invest Dermatol. 2005. 125:510–520.

68. Hachem JP, Crumrine D, Fluhr J, Brown BE, Feingold KR, Elias PM. pH directly regulates epidermal permeability barrier homeostasis, and stratum corneum integrity/cohesion. J Invest Dermatol. 2003. 121:345–353.

69. Rippke F, Schreiner V, Doering T, Maibach HI. Stratum corneum pH in atopic dermatitis: impact on skin barrier function and colonization with Staphylococcus Aureus. Am J Clin Dermatol. 2004. 5:217–223.
70. Demerjian M, Hachem JP, Tschachler E, Denecker G, Declercq W, Vandenabeele P, et al. Acute modulations in permeability barrier function regulate epidermal cornification: role of caspase-14 and the protease-activated receptor type 2. Am J Pathol. 2008. 172:86–97.

71. Schmid-Wendtner MH, Korting HC. The pH of the skin surface and its impact on the barrier function. Skin Pharmacol Physiol. 2006. 19:296–302.

72. Yosipovitch G, Papoiu AD. What causes itch in atopic dermatitis? Curr Allergy Asthma Rep. 2008. 8:306–311.

73. Jeong SK, Kim HJ, Youm JK, Ahn SK, Choi EH, Sohn MH, et al. Mite and cockroach allergens activate protease-activated receptor 2 and delay epidermal permeability barrier recovery. J Invest Dermatol. 2008. 128:1930–1939.

74. Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002. 347:1151–1160.

75. Nomura I, Gao B, Boguniewicz M, Darst MA, Travers JB, Leung DY. Distinct patterns of gene expression in the skin lesions of atopic dermatitis and psoriasis: a gene microarray analysis. J Allergy Clin Immunol. 2003. 112:1195–1202.

76. Nomura I, Goleva E, Howell MD, Hamid QA, Ong PY, Hall CF, et al. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol. 2003. 171:3262–3269.

77. Howell MD, Boguniewicz M, Pastore S, Novak N, Bieber T, Girolomoni G, et al. Mechanism of HBD-3 deficiency in atopic dermatitis. Clin Immunol. 2006. 121:332–338.

78. Howell MD, Novak N, Bieber T, Pastore S, Girolomoni G, Boguniewicz M, et al. Interleukin-10 downregulates anti-microbial peptide expression in atopic dermatitis. J Invest Dermatol. 2005. 125:738–745.

79. Ong PY, Leung DY. Immune dysregulation in atopic dermatitis. Curr Allergy Asthma Rep. 2006. 6:384–389.

80. Aberg KM, Radek KA, Choi EH, Kim DK, Demerjian M, Hupe M, et al. Psychological stress downregulates epidermal antimicrobial peptide expression and increases severity of cutaneous infections in mice. J Clin Invest. 2007. 117:3339–3349.

81. Kuo IH, Carpenter-Mendini A, Yoshida T, McGirt LY, Ivanov AI, Barnes KC, et al. Activation of epidermal toll-like receptor 2 enhances tight junction function: implications for atopic dermatitis and skin barrier repair. J Invest Dermatol. 2013. 133:988–998.

82. Lee SE, Choi Y, Kim SE, Noh EB, Kim SC. Differential effects of topical corticosteroid and calcineurin inhibitor on the epidermal tight junction. Exp Dermatol. 2013. 22:59–61.

83. Kurahashi R, Hatano Y, Katagiri K. IL-4 suppresses the recovery of cutaneous permeability barrier functions in vivo. J Invest Dermatol. 2008. 128:1329–1331.

84. Hatano Y, Terashi H, Arakawa S, Katagiri K. Interleukin-4 suppresses the enhancement of ceramide synthesis and cutaneous permeability barrier functions induced by tumor necrosis factor-alpha and interferon-gamma in human epidermis. J Invest Dermatol. 2005. 124:786–792.

85. Kim BE, Leung DY, Boguniewicz M, Howell MD. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin Immunol. 2008. 126:332–337.

86. Albanesi C, Fairchild HR, Madonna S, Scarponi C, De Pita O, Leung DY, et al. IL-4 and IL-13 negatively regulate TNF-alpha- and IFN-gamma-induced beta-defensin expression through STAT-6, suppressor of cytokine signaling (SOCS)-1, and SOCS-3. J Immunol. 2007. 179:984–992.

87. Howell MD, Fairchild HR, Kim BE, Bin L, Boguniewicz M, Redzic JS, et al. Th2 cytokines act on S100/A11 to downregulate keratinocyte differentiation. J Invest Dermatol. 2008. 128:2248–2258.

88. Ziegler SF, Liu YJ. Thymic stromal lymphopoietin in normal and pathogenic T cell development and function. Nat Immunol. 2006. 7:709–714.

89. Sano Y, Masuda K, Tamagawa-Mineoka R, Matsunaka H, Murakami Y, Yamashita R, et al. Thymic stromal lymphopoietin expression is increased in the horny layer of patients with atopic dermatitis. Clin Exp Immunol. 2013. 171:330–337.

90. Chamlin SL, Kao J, Frieden IJ, Sheu MY, Fowler AJ, Fluhr JW, et al. Ceramide-dominant barrier repair lipids alleviate childhood atopic dermatitis: changes in barrier function provide a sensitive indicator of disease activity. J Am Acad Dermatol. 2002. 47:198–208.

91. Wood LC, Elias PM, Calhoun C, Tsai JC, Grunfeld C, Feingold KR. Barrier disruption stimulates interleukin-1 alpha expression and release from a pre-formed pool in murine epidermis. J Invest Dermatol. 1996. 106:397–403.

92. Wood LC, Stalder AK, Liou A, Campbell IL, Grunfeld C, Elias PM, et al. Barrier disruption increases gene expression of cytokines and the 55 kD TNF receptor in murine skin. Exp Dermatol. 1997. 6:98–104.

93. Onoue A, Kabashima K, Kobayashi M, Mori T, Tokura Y. Induction of eosinophil- and Th2-attracting epidermal chemokines and cutaneous late-phase reaction in tape-stripped skin. Exp Dermatol. 2009. 18:1036–1043.

94. Briot A, Deraison C, Lacroix M, Bonnart C, Robin A, Besson C, et al. Kallikrein 5 induces atopic dermatitis-like lesions through PAR2-mediated thymic stromal lymphopoietin expression in Netherton syndrome. J Exp Med. 2009. 206:1135–1147.

95. Takai T. TSLP expression: cellular sources, triggers, and regulatory mechanisms. Allergol Int. 2012. 61:3–17.

96. Hatano Y, Katagiri K, Arakawa S, Fujiwara S. Interleukin-4 depresses levels of transcripts for acid-sphingomyelinase and glucocerebrosidase and the amount of ceramide in acetone-wounded epidermis, as demonstrated in a living skin equivalent. J Dermatol Sci. 2007. 47:45–47.

97. Cornelissen C, Marquardt Y, Czaja K, Wenzel J, Frank J, Luscher-Firzlaff J, et al. IL-31 regulates differentiation and filaggrin expression in human organotypic skin models. J Allergy Clin Immunol. 2012. 129:426–433.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download