Abstract
Objective
Decompressive craniectomy is widely used in cases of uncontrolled intracranial hypertension, including traumatic brain injury or acute stroke. Physiological monitorings, such as intracranial pressure or electroenecephalography (EEG) are critical for patients in the acute phase. We retrospectively reviewed our experience of continuous electrocorticography (ECoG) monitoring by subdural strip electrode in patients who performed decompressive craniectomy and assessed its clinical efficacy.
Methods
Patients who underwent decompressive craniectomy because of severe intracranial hypertension were included. 4 Channel strip electrodes were inserted on the frontal cortex before closure. 24-hour continuous monitoring of ECoG was done to identify abnormal electrical activity. The level of consciousness was assessed according to Glasgow Coma Scale (GCS). In patients with malignant intracranial hypertension, barbiturate coma therapy was considered.
Results
Fifteen patients (9 men and 6 women) were included and the mean age was 55.7 years (from 17 to 80). The initial mean GCS score was 7.9 (from 3 to 14). In six out of fifteen patients, abnormal spike activities were identified, and one of these six patients was diagnosed as nonconvulsive status epilepticus (NCSE). Cortical spreading depression (CSD) was suspected in five. Three patients underwent barbiturate coma therapy and ECoG monitoring of these patients showed typical burst suppression pattern, which was used for indicator of therapeutic level. The mean duration of strip electrode and ECoG monitoring was 3.5 days, and there was no complication.
References
1. Chen HI, Malhotra NR, Oddo M, Heuer GG, Levine JM, LeRoux PD. Barbiturate infusion for intractable intracranial hypertension and its effect on brain oxygenation. Neurosurgery. 63:880–886. ; discussion 886–887,. 2008.

2. Cottenceau V, Petit L, Masson F, Guehl D, Asselineau J, Cochard JF, et al. The use of bispectral index to monitor barbiturate coma in severely brain-injured patients with refractory intracranial hypertension. Anesth Analg. 107:1676–1682. 2008.

3. Fountas KN, Smith JR. Subdural electrode-associated complications: a 20-year experience. Stereotact Funct Neurosurg. 85:264–272. 2007.

4. Hartings JA, Strong AJ, Fabricius M, Manning A, Bhatia R, Dreier JP, et al. Spreading depolarizations and late secondary insults after traumatic brain injury. J Neurotrauma. 26:1857–1866. 2009.

5. Jirsch J, Hirsch LJ. Nonconvulsive seizures: developing a rational approach to the diagnosis and management in the critically ill population. Clin Neurophysiol. 118:1660–1670. 2007.
6. Jordan KG. Nonconvulsive status epilepticus in acute brain injury. J Clin Neurophysiol. 16:332–340. ; discussion 353,. 1999.

7. Kennedy JD, Gerard EE. Continuous EEG monitoring in the intensive care unit. Curr Neurol Neurosci Rep. 12:419–428. 2012.

8. Lauritzen M, Dreier JP, Fabricius M, Hartings JA, Graf R, Strong AJ. Clinical relevance of cortical spreading depression in neurological disorders: migraine, malignant stroke, subarachnoid and intracranial hemorrhage, and traumatic brain injury. J Cereb Blood Flow Metab. 31:17–35. 2011.

10. Ronne-Engstrom E, Winkler T. Continuous EEG monitoring in patients with traumatic brain injury reveals a high incidence of epileptiform activity. Acta Neurol Scand. 114:47–53. 2006.

11. Van Gompel JJ, Worrell GA, Bell ML, Patrick TA, Cascino GD, Raffel C, et al. Intracranial electroencephalography with subdural grid electrodes: techniques, complications, and outcomes. Neurosurgery. 63:498–505. ; discussion 505–506,. 2008.
FIGURE 1.
A: 4-channel strip electrode. The width of electrode is 5 mm and the gap between electrodes is 10 mm. B: Skull X-ray (lateral view) after decompressive craniectomy. Subdural strip electrode (arrow) was inserted.
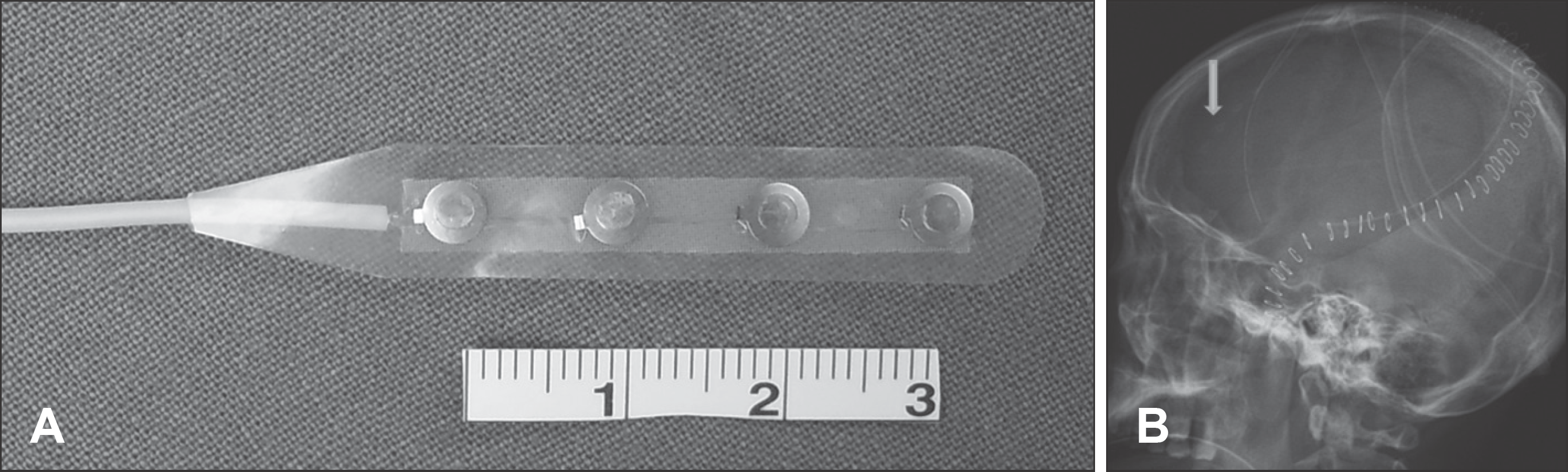
FIGURE 2.
A: Nonconvulsive status epilepticus in patient with acute subdural hematoma (Patient No.10). Abnormal fast spiking activities are shown in all channels when patient showed ictal nystagmus and decrease of consciousness. B: Cortical spreading depression in patient with subarachnoid hemorrhage (Patient No.2). Time-compressed tracing shows depressed ECoG activity (arrowhead), about 60% reduction of amplitude, evolved over 30 seconds and lasted for about 5 minutes in upper row. This is followed by a similar change in lower row about 4 minutes later. The distance of each contact of strip electrode is 10 mm, so the propagation rate is about 2 mm/min. C: Burst-suppression pattern of ECoG activity in patient with barbiturate coma therapy (Patient No. 1). Bursting activities (arrows) are shown in all channels after several seconds period of suppressed ECoG. ECoG: electrocorticogram.

TABLE 1.
Demographics of the study population
| Case no. | Sex | Age | Diagnosis | Initial GCS∗ | Duration (days) | Coma therapy | ECoG finding |
|---|---|---|---|---|---|---|---|
| 1 | M | 54 | ASDH | 11 (2–4–5) | 5 | (+) | CSD |
| 2 | F | 54 | SAH | 11 (3–3–5) | 3 | – | CSD |
| 3 | M | 24 | Lymphoma | 4 (1–1–2) | 4 | (+) | NS |
| 4 | M | 46 | Infarction | 11 (3–3–5) | 3 | – | CSD |
| 5 | F | 63 | SAH | 14 (3–5–6)† | 2 | – | Spike (+) |
| 6 | M | 57 | Infarction | 11 (3–3–5) | 2 | – | NS |
| 7 | F | 66 | Infarction | 7 (1–1–5) | 3 | – | Spike (+), CSD |
| 8 | F | 17 | Encephalitis | 3 (1–1–1) | 4 | (+) | Spike (+) |
| 9 | M | 43 | ICH | 4 (1–1–2) | 3 | – | Spike (+), CSD |
| 10 | F | 80 | ASDH | 4 (1–1–2) | 3 | – | Status epilepticus |
| 11 | M | 67 | ICH | 4 (1–1–2) | 5 | – | NS |
| 12 | F | 71 | ASDH | 8 (2–1–5) | 4 | – | Spike (+) |
| 13 | M | 66 | ASDH | 4 (1–1–2) | 2 | – | NS |
| 14 | M | 53 | Infraction | 11 (3–3–5) | 2 | – | NS |
| 15 | M | 74 | ASDH | 11 (3–3–5) | 3 | – | NS |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download