This article has been
cited by other articles in ScienceCentral.
Abstract
We report a case involving the development of a delayed acute subdural hematoma (ASDH) after trauma, with the absence of any abnormal radiological and clinical findings at initial examination. A 54-year-old male visited the emergency department after a minor trauma. The patient only complained of mild headache after head injury. He presented no abnormal findings on neurological examination, and brain computed tomography (CT) did not show any intracranial lesion or skull fractures. However, he developed seizure with disorientation eight hours after trauma, and ASDH with midline shift was found during a follow-up CT. He recovered without neurological deficits after immediate primary care and admission to the neurosurgery department. On serial follow-up CT images, a gradually increasing mass effect of hematoma was detected, and removed by craniotomy. The patient recovered without neurologic deficits.
Go to :

Keywords: Acute subdural hematoma, Surgical decompression, Traumatic brain injury
Introduction
Most of minor head injuries present absence of intracranial lesion on computed tomography (CT) and negative neurological findings, however, developing life-threatening intracranial complications needing neurosurgical intervention are rarely exist.
Delayed intracranial hematoma has been documented in patients with coagulation disorder or patients taking anticoagulant or antiplatelet therapy in occasional literature.
5) Most of the hematoma have been described regarding delayed development of traumatic intraparenchymal or extradural hemorrhages.
245) But development of delayed acute subdural hematoma (DASH) in patients without having coagulation disorder or any other risk factors is rare and not fully understood. We are reporting a case of DASH on subsequent CT after minor head injury in a patient who did not show any radiological and clinical abnormality at initial state. And we briefly review the literature and physiology of DASH.
Go to :

Case Report
A 54-year-old male patient visited to emergency department for minor head injury after he slipped down on stairs. He underwent brain CT scan on approximately 2 hours after trauma (
Figure 1A). He presented no clinical abnormality on initial neurologic examinations except mild headache; his vitals were stable, Glasgow Coma Scale (GCS) score was 15 (E4V5M6), pupils were normal bilaterally, and there was no history of loss of consciousness. Also, he had no recent history of any medical treatments or other hematologic disorders, hypertension, diabetes mellitus and alcoholism. No bony fracture, intracranial lesion, or mass effects were detected on CT scan. He was monitored and treated for symptomatic control in the emergency department. Then, the sudden onset of generalized tonic-clonic seizure was developed on 8 hours after trauma. Newly found acute subdural hematoma (ASDH) was detected in left cerebral convexity on subsequent brain CT and midline was shifted less than 2 mm (
Figure 1B). There was no history of additional trauma while he was treated in the emergency department and no evidence of underlying medical dysfunction in laboratory findings. On further examination, hematologic test including coagulation profile and peripheral blood morphology was confirmed within normal range. The patient referred to neurosurgery department and admitted to intensive unit care. We followed-up repeated CT 24 hours after admission; there was no change of amount of hematoma. Though he sustained moderate headache, vitals were stable and GCS score was 15 without neurological deficit. The volume of ASDH did not change during serial CT on 3 and 5 days of admission. However, on 8 days of admission, upper and lower extremity motor grade of patient were deteriorated to grade 4 on right side. CT scan disclosed increasing amount of mixed-dense subdural hematoma (SDH) with mass effect (
Figure 2A). There was no evidence of acute cerebral infarction or other intracranial lesions except SDH on diffusion weighted magnetic resonance image (
Figure 2B). The patient became presenting intense headache and disorientation. Emergent surgical intervention of craniotomy with hematoma removal was performed and semisolid blood clot was evacuated. On the 1st post-operative day, CT showed no mass effect with midline recovery (
Figure 3A). The patient's motor grade was nearly recovered to grade 5; however, recurrent ASDH was detected on follow-up CT scan on 3rd postoperative day (
Figure 3B). Surgical intervention of craniotomy and hematoma evacuation was re-conducted in a same manner. The patient was discharged on the 10th postoperative day with GCS score of 15 and Glasgow Outcome Scale of 5 (
Figure 3C).
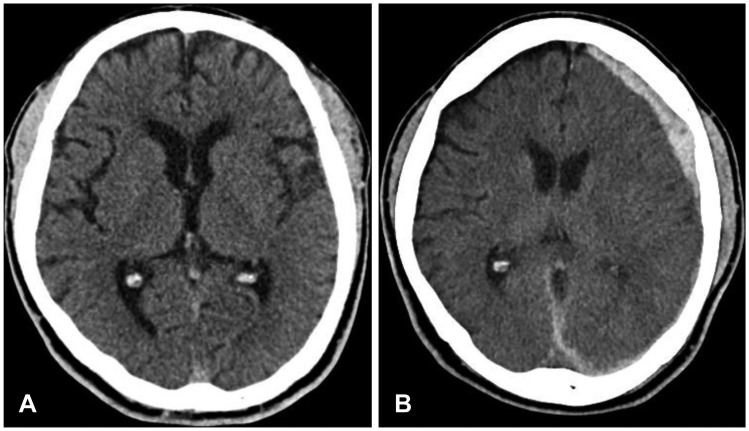 | FIGURE 1(A) Initial computed tomography (CT) shows no abnormalities. (B) Follow-up CT shows acute subdural hematoma on left cerebral convexity on 8 hours after trauma. 
|
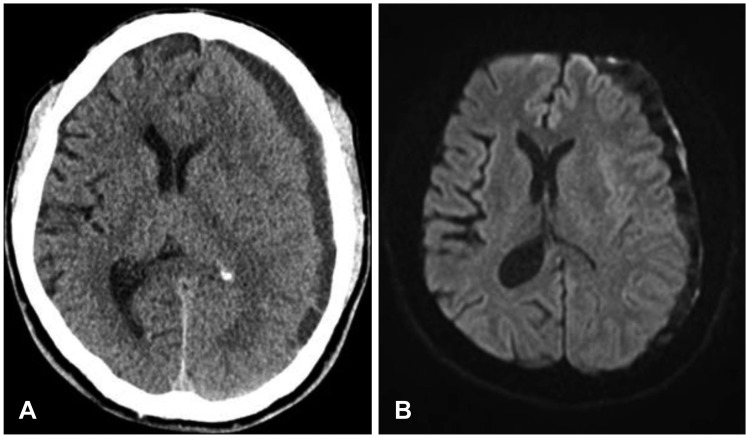 | FIGURE 2(A) Computed tomography scan disclose increasing mass effect of hematoma and midline shift of approximately 10 mm. (B) Diffusion weighted magnetic resonance image shows mixed density of delayed acute subdural hematoma. 
|
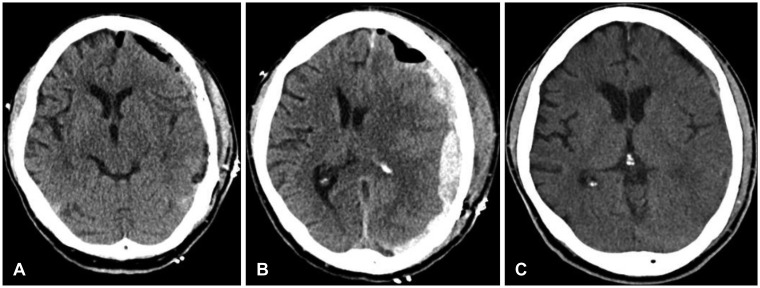 | FIGURE 3 (A) Postoperative computed tomography (CT) after hematoma evacuation shows the recovered midline. (B) CT reveals recurrent acute subdural hematoma on 3rd postoperative day (C) CT scan shows no additional hematoma or other abnormal findings at discharge. 
|
Go to :

Discussion
Mild traumatic brain injury (mTBI) is defined as a head injury for which GCS score is 13 to 15. The majority of patients with mTBI have uneventful recovery; however, misdiagnosis can result in death, prolonged vegetative states, or significant disability.
4) Every patient with a GCS score less than 15 after mTBI is recommended to admit to hospital for close monitoring
8) and any abnormal findings including post-traumatic amnesia, coagulopathy, abnormal neurological examination result, or intracerebral abnormalities should not be overlooked.
In previous literature, delayed traumatic intracranial hemorrhages (DTICH) were described and most were intraparenchymal and extradural hematoma. But the delayed development of ASDH is not frequently reported.
238) According to Gudeman et al.
3), DTICH was defined as subsequently developed high-density parenchymal lesion in area previously normal or with negligible abnormality. Fukamachi et al.
2) describes hematoma that appears subsequently, where the initial CT scan could not elicit any abnormalities. Patients with risk factors like alcoholism, coagulation disorder, taking anticoagulant or antiplatelet, abnormal liver function, diabetes mellitus, hypertension, and other associative underlying diseases, have vulnerabilities against hemorrhage in previous reports. In addition, any underlying intracranial lesions, such as a tumor or aneurysm, had been described as risk factors to have intracranial hemorrhage. Shabani et al.
8) said that the prevalence of DASH was much higher in patients who were taking anticoagulation or antiplatelet medication, or both, compared with those who were not taking anything (80% vs. 20%). In our case report, any of the above risk factors was not listed in our patient record and laboratory findings.
ASDH commonly occurs from tearing of bridging veins or from injury to cortical arteries or veins. Bridging veins usually tend to have a short intradural course prior to opening into the superior sagittal sinus and may anatomically be vulnerable to shearing force causing SDH.
6) Among few reported cases of DASH, Nath et al.
7) hypothesized cause of DASH as shearing of the brain within the cranial cavity resulting in microvascular injury of the bridging vein wall: perivascular degenerative changes result in hypoxic damage and necrosis of vessel wall, which is causative of subsequent bleeding. Theses microvasculature injuries and perivascular tissue damages could lead to ischemic brain damage which related to progressive brain swelling and increase in the intracranial pressure. Subsequently, concomitant elevation of venous pressure and terminal vein pressure could occur. Vasa vasorum is a network of blood vessel that supplies the walls of arteries and veins to provide blood supply and nourishment for tunica media and tunica adventitia. As described by Zervas et al.
9), the cerebral blood vessels are devoid of vasa vasorum. Instead, the cerebral vessels contain a rete vasorum in the adventitia that is permeable to large proteins and is in continuity with the subarachnoid space. Injury to this rete vasorum may cause hypoxic injury to the vessel wall, leading to degeneration and disruption of the vessel wall, with subsequent bleeding which needs further histopathological evaluation.
7) The other hypotheses were spontaneous vascular rupture, resulting from progressive vascular degeneration, the local metabolites causing vascular wall injury,
1) vasoparalysis is resulting from local hypoxia,
3) and increased intracranial pressure causing venous congestion produced by Valsalva effect.
7) Matsumura et al.
5) described that the Valsalva maneuver might have caused increased intracranial pressure, hypercapnia, and subsequent venous congestion, resulting in SDH because of a rupture of coalescent vessels.
Although DASH has not been fully understood, DASH could be triggered by direct injury of rupture of bridging veins or small cortical arteries after a period of time. However, considering previous reviews, the hypothesis can be suggested in our case that shearing of the brain within intracranial cavity lead to microvascular injury of the bridging vein walls, and progressive degenerative changes caused by hypoxic and inflammatory damages. This disruption of microvasculature could result in substantive vascular injury and concomitant perivascular ischemic brain damage in the cortex underneath the SDH could expect brain swelling and increase ICP, which is, in turn, possibly leading to escalation of venous pressure or spontaneous vascular rupture on delayed phase.
Go to :

Conclusion
Delayed onset of SDH is a rare, but reported entity that should always be considered. For physician, it is important to notice symptoms in the early posttraumatic period. As presented in our case, clinical observation after head injury is recommended and necessity of follow-up CT or neurologic examination should not be overlooked.
Go to :


