Introduction
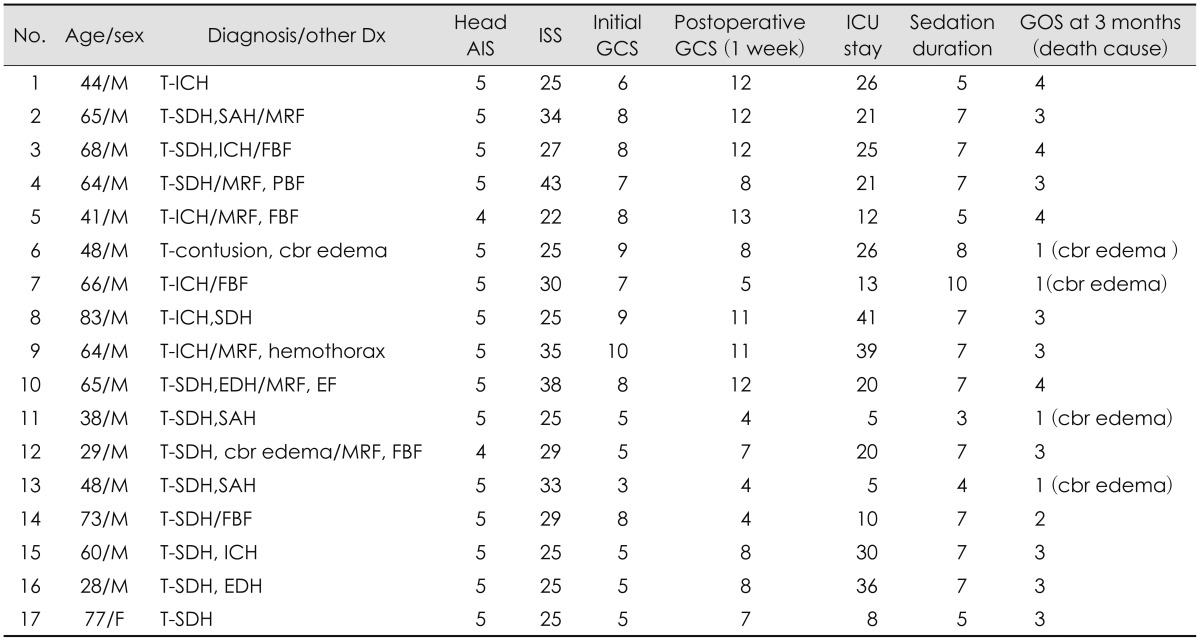
Importance of post-operative management of intracranial pressure (ICP) and cerebral perfusion pressure (CPP) in severe traumatic brain injury (TBI) patients cannot be emphasized enough since it has direct influence over patient treatment outcome.
15) In order to reach the appropriate CPP and ICP, meticulous control of mean arterial pressure (MAP), is key, however, this is usually a difficult process since severe TBI patients usually have combined multiple injuries rather than single isolated head injuries. The complications arising from fluid therapy to control the ICP and MAP, such as volume depletion, pneumonia, pulmonary edema due to volume overload and congestive heart failure (CHF) secondary acute renal failure (ARF) may result in high patient's morbidity and mortality. Up to now, most effective monitoring methods for MAP regulation are continuous arterial-line monitoring through an arterial catheter and central venous pressure (CVP) through a central catheter.
9)
Recently, a new cardiac output (CO) device which accurately measures CO and MAP is being widely used for intensive care unit (ICU) critical care.
1118) Dynamic parameters of fluid responsiveness in CO monitoring device-stroke volume variation (SVV) and stroke volume index (SVI)-can be obtained from analysis of arterial waveform.
12) Differences in systolic pressure and pulse pressure are generated by changes in stroke volume during respiratory cycle. The amount of variation (SVV) correlates well with volume responsiveness.
17) Also, SVI is known to be represented as the measure of cardiac performance.
12) Therefore, we can estimate the patient's volume status and cardiac function by using these two parameters. In this study, we aimed to maintain optimal ICP and reduce fluid therapy related complications in severe TBI patients who received CPP, MAP control by using ICP monitoring and arterial line based CO monitoring simultaneously.
Go to :

Discussion
The brain trauma foundation has established guideline for ICP monitoring in severe TBI.
619) These studies demonstrated that superior survival was observed in severe TBI patients with ICP monitoring. However, other studies revealed that use of the ICP monitoring in isolated severe TBI had no survival benefit and was associated with occurrences of more complications which increased utilization of hospital resources.
12) There are three methods for lowering ICP via vasoconstriction without reducing cerebral blood flow (CBF). First, decreasing blood viscosity in patients with intact viscosity autoregulation results in decreased ICP without significant changes in CBF. The sustained ICP lowering effects of hypertonic saline and mannitol likely result in part from the impact on blood viscosity.
41416) Second, in patients with intact pressure autoregulation, increasing CPP will trigger vasoconstriction which decreases CBV and ICP; CBF remains constant in these circumstances.
15) Third, sedation (e.g., propofol, pentobarbiturate infusion) reduces cerebral metabolic rate of oxygen, which not only protects cells from secondary injuries and insults, but also reduces ICP through metabolic autoregulatory pathways. If the patients undergo sedation therapy, sudden drop of blood pressure can often occurs, lowering MAP which results in imbalance of CPP causing uncontrolled brain edema or vicious cycle. Therefore, many guidelines suggested that comatose patients with severe TBI should be mechanically ventilated and monitored with an invasive arterial line, a central venous catheter, and an ICP pressure probe.
9)
Usually severe TBI patients have combined multiple traumatic injuries which makes treatment very complicated. In order to maintain adequate CBF in severe TBI patients accurate monitoring of ICP and CPP is crucial. An ICP monitor can provide objective and exact values in order to maintain the CBF in such patients. However, the first goal of severe TBI is normalization of brain tissue oxygenation and CPP. If patients suffered multiple traumas which could result in major blood losses such as hemoperitoneum, hemothorax or major fracture of extremities, maintaining an optimum MAP and CPP is very challenging because MAP value is reversely proportionate to ICP value.
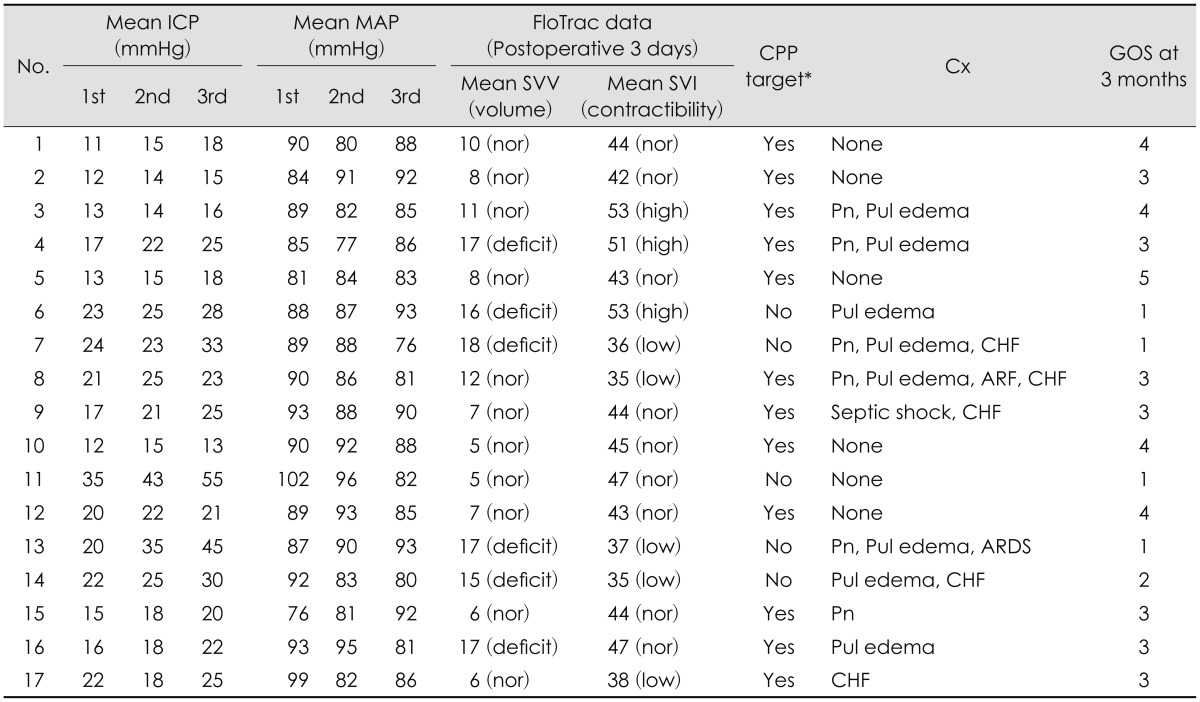
19) In this situation, volume status and cardiac contractibility in patients are important indicators for optimizing systemic treatment such as fluid resuscitation or injection inotropics or diuretics. Also with the introduction of advanced CO monitoring, it is possible to continuously monitor MAP and CO which enables physicians to deduce the change in CPP. Therefore, the goal of advanced neuromonitoring in patients with severe brain injury should be to allow early detection of fluid therapy related complications such as pneumonia, pulmonary edema, ARDS, ARF, CHF and electrolyte imbalance etc.
Numerous supportive methods have been introduced in order to optimize treatment in TBI patients
6910) In the past, CVP or body weights were the only methods to notify volume status of the patient indirectly.
9) Recently, various methods have been introduced to determine volume status with safe and accuracy.
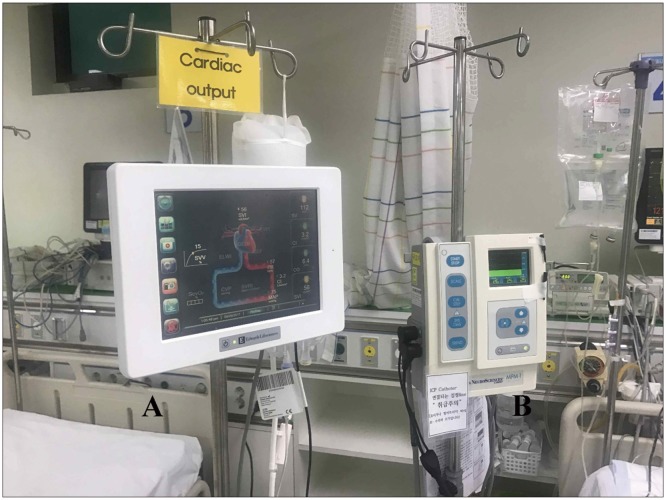
9101112) These recent device are more accurate and easier to manipulate for physicians to evaluate patients' volume status and cardiac function. One recently developed CO monitoring system (Vigileo™; Edwards Life sciences) is also based on arterial pulse contour analysis. A special blood flow sensor (FloTrac), which is connected to an arterial line (radial, brachial, axillary or femoral arteries) is needed with no external calibration is necessary.
111318) This device calculates CO on a continuous basis (every 20s) by multiplying pulse rate by calculated stroke volume which analyzed the impact of vascular tone on pressure and adjustment for actual vascular tone based on waveform analysis.
5) SVV is the ability of the heart to increase stroke volume in response to an increase in preload. It is estimated dynamic parameters volume challenge maneuver, respiratory variation.
1012) Based on this, a mathematical model was developed. Several studies have been performed concerning the accuracy of Vigileo CO monitoring that include a variety of patients with different software versions of the device.
1718) Other limitations of this device include lack of validation in a pediatric population, total dependence on arterial line waveform for accurate calculations, the presence of dysrhythmias or improper dampening from kinking, shock state and significant valvular disease that can prevent accurate results.
20)
Low SVV is good indicator of fluid resuscitation for TBI patients under sedation and a ventilator. After fluid resuscitation, systemic hemodynamic resuscitation should always precede brain targeted interventions.
10) Assessment of fluid responsiveness may be possible by following global end diastolic volume index (GEDVI), SVV, and pulse pressure variation. CVP is a poor indicator for fluid responsiveness or intravascular volume status. SVV greater than 10% to 14% and GEDVI less the 600 mL/m
2 generally indicate that the patients will respond to a fluid challenge with either 500 mL of crystalloid or 250 mL of colloid solution.
10)
The sudden development of hypoxemic respiratory failure following a catastrophic central nervous system event, which cannot be attributed to other causes of ARDS, is the only universally agreed upon characteristic of neurogenic pulmonary edema (NPE).
78) In patients with TBI, the incidence of NPE has been estimated to be up to 20%.
3) It appears that the clinical manifestations of this surge may vary depending on the individual circumstance. Although this article does not include the diagnosis and early detection of ARDS or NPE, it would be considered an important issue in our following paper.
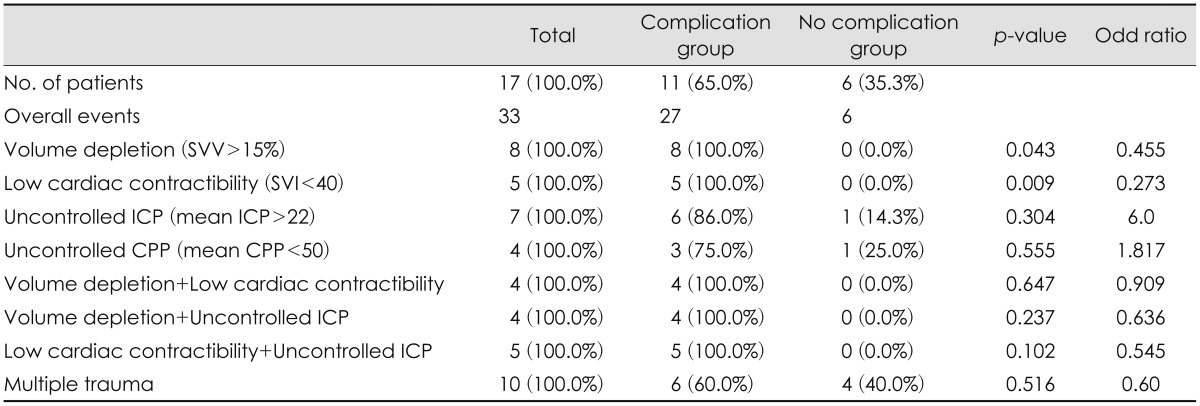
Although our study is based upon retrospective data, there are no reports to evaluate for fluid therapy related complications in severe TBI patients with CO and ICP monitor at the same time. The mortality of the patients due to complication during ICU care rather than one due to failure of control ICP and progressive hemorrhage can be very unfortunate event for neurosurgeons. Therefore, as we have seen in our study, the dual monitor setups would be a very useful alternative treatment to maintain optimal CPP, ICP and minimal fluid therapy related complications.
There are some limitations in this study. First, it is a retrospective study design with a small population. Second, the mean value of parameter in CO and ICP monitor is not accurate one due to a flow recording continuously and subdural type ICP monitor is less accurate than intraparenchymal or intraventricular type. Third, it is unreliable due to there are many heterogeneous factors in investigating one related to complications. Last, it is difficult to distinguish between NPE and fluid related pulmonary edema. Therefore, further studies will be required to ascertain the long term follow up results and recruit more cases of TBI patients using dual monitor and compare with control group for more accurate effect of combined monitor.
Go to :





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download