1. Al-Tamimi YZ, Sinha P, Chumas PD, Crimmins D, Drake J, Kestle J, et al. Ventriculoperitoneal shunt 30-day failure rate: a retrospective international cohort study. Neurosurgery. 2014; 74:29–34. PMID:
24089046.

2. Ameli PA, Madan M, Chigurupati S, Yu A, Chan SL, Pattisapu JV. Effect of acetazolamide on aquaporin-1 and fluid flow in cultured choroid plexus. Acta Neurochir Suppl. 2012; 113:59–64. PMID:
22116425.

3. Bloch O, Auguste KI, Manley GT, Verkman AS. Accelerated progression of kaolin-induced hydrocephalus in aquaporin-4-deficient mice. J Cereb Blood Flow Metab. 2006; 26:1527–1537. PMID:
16552421.

4. Drake JM, Kulkarni AV, Kestle J. Endoscopic third ventriculostomy versus ventriculoperitoneal shunt in pediatric patients: a decision analysis. Childs Nerv Syst. 2009; 25:467–472. PMID:
19139908.

5. Filippidis AS, Kalani MY, Rekate HL. Hydrocephalus and aquaporins: the role of aquaporin-4. Acta Neurochir Suppl. 2012; 113:55–58. PMID:
22116424.

6. Ivkovic M, Reiss-Zimmermann M, Katzen H, Preuss M, Kovanlikaya I, Heier L, et al. MRI assessment of the effects of acetazolamide and external lumbar drainage in idiopathic normal pressure hydrocephalus. Fluids Barriers CNS. 2015; 12:9. PMID:
25928394.

7. Johanson CE, Duncan JA 3rd, Klinge PM, Brinker T, Stopa EG, Silverberg GD. Multiplicity of cerebrospinal fluid functions: New challenges in health and disease. Cerebrospinal Fluid Res. 2008; 5:10. PMID:
18479516.

8. Juhler M. Animal models of hydrocephalus. In : Rigamonti D, editor. Adult hydrocephalus. New York, NY: Cambridge University Press;2014. p. 28–35.
9. Kadrian D, van Gelder J, Florida D, Jones R, Vonau M, Teo C, et al. Long-term reliability of endoscopic third ventriculostomy. Neurosurgery. 2005; 56:1271–1278. PMID:
15918943.

10. Kalani MY, Filippidis AS, Rekate HL. Hydrocephalus and aquaporins: the role of aquaporin-1. Acta Neurochir Suppl. 2012; 113:51–54. PMID:
22116423.

11. Kowarik MC, Soltys J, Bennett JL. The treatment of neuromyelitis optica. J Neuroophthalmol. 2014; 34:70–82. PMID:
24531318.

12. Li J, McAllister JP 2nd, Shen Y, Wagshul ME, Miller JM, Egnor MR, et al. Communicating hydrocephalus in adult rats with kaolin obstruction of the basal cisterns or the cortical subarachnoid space. Exp Neurol. 2008; 211:351–361. PMID:
18433747.

13. Longatti P, Basaldella L, Orvieto E, Dei Tos A, Martinuzzi A. Aquaporin(s) expression in choroid plexus tumours. Pediatr Neurosurg. 2006; 42:228–233. PMID:
16714863.

14. Mao X, Enno TL, Del Bigio MR. Aquaporin 4 changes in rat brain with severe hydrocephalus. Eur J Neurosci. 2006; 23:2929–2936. PMID:
16819982.
15. McAllister JP, Eskandari R, Limbrick DD Jr. Experimental hydrocephalus. In : Winn HR, editor. Youmans & Winn neurological surgery. Philadelphia, PA: Elsevier;2017. p. 1614.
16. Nagelhus EA, Ottersen OP. Physiological roles of aquaporin-4 in brain. Physiol Rev. 2013; 93:1543–1562. PMID:
24137016.

17. Oshio K, Watanabe H, Song Y, Verkman AS, Manley GT. Reduced cerebrospinal fluid production and intracranial pressure in mice lacking choroid plexus water channel Aquaporin-1. FASEB J. 2005; 19:76–78. PMID:
15533949.

18. Papadopoulos MC, Bennett JL, Verkman AS. Treatment of neuromyelitis optica: state-of-the-art and emerging therapies. Nat Rev Neurol. 2014; 10:493–506. PMID:
25112508.

19. Papadopoulos MC, Manley GT, Krishna S, Verkman AS. Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. FASEB J. 2004; 18:1291–1293. PMID:
15208268.

20. Papadopoulos MC, Verkman AS. Aquaporin water channels in the nervous system. Nat Rev Neurosci. 2013; 14:265–277. PMID:
23481483.

21. Park MK, Kim M, Park KS, Park SH, Hwang JH, Hwang SK. A retrospective analysis of ventriculoperitoneal shunt revision cases of a single institute. J Korean Neurosurg Soc. 2015; 57:359–363. PMID:
26113963.

22. Paul L, Madan M, Rammling M, Chigurupati S, Chan SL, Pattisapu JV. Expression of aquaporin 1 and 4 in a congenital hydrocephalus rat model. Neurosurgery. 2011; 68:462–473. PMID:
21135737.

23. Pollay M. Overview of the CSF dual outflow system. Acta Neurochir Suppl. 2012; 113:47–50. PMID:
22116422.

24. Preston GM, Agre P. Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family. Proc Natl Acad Sci U S A. 1991; 88:11110–11114. PMID:
1722319.

25. Reddy GK, Bollam P, Caldito G. Ventriculoperitoneal shunt surgery and the risk of shunt infection in patients with hydrocephalus: long-term single institution experience. World Neurosurg. 2012; 78:155–163. PMID:
22120565.

26. Shen XQ, Miyajima M, Ogino I, Arai H. Expression of the water-channel protein aquaporin 4 in the H-Tx rat: possible compensatory role in spontaneously arrested hydrocephalus. J Neurosurg. 2006; 105:459–464. PMID:
17184078.

27. Smith ZA, Moftakhar P, Malkasian D, Xiong Z, Vinters HV, Lazareff JA. Choroid plexus hyperplasia: surgical treatment and immunohistochemical results. Case report. J Neurosurg. 2007; 107:255–262. PMID:
17918538.
28. Stone JJ, Walker CT, Jacobson M, Phillips V, Silberstein HJ. Revision rate of pediatric ventriculoperitoneal shunts after 15 years. J Neurosurg Pediatr. 2013; 11:15–19. PMID:
23101557.

29. Tait MJ, Saadoun S, Bell BA, Papadopoulos MC. Water movements in the brain: role of aquaporins. Trends Neurosci. 2008; 31:37–43. PMID:
18054802.

30. Verkman AS. Aquaporins in clinical medicine. Annu Rev Med. 2012; 63:303–316. PMID:
22248325.

31. Wagshul ME, McAllister JP, Rashid S, Li J, Egnor MR, Walker ML, et al. Ventricular dilation and elevated aqueductal pulsations in a new experimental model of communicating hydrocephalus. Exp Neurol. 2009; 218:33–40. PMID:
19348801.

32. Whitelaw A, Aquilina K. Management of posthaemorrhagic ventricular dilatation. Arch Dis Child Fetal Neonatal Ed. 2012; 97:F229–F233. PMID:
21289015.

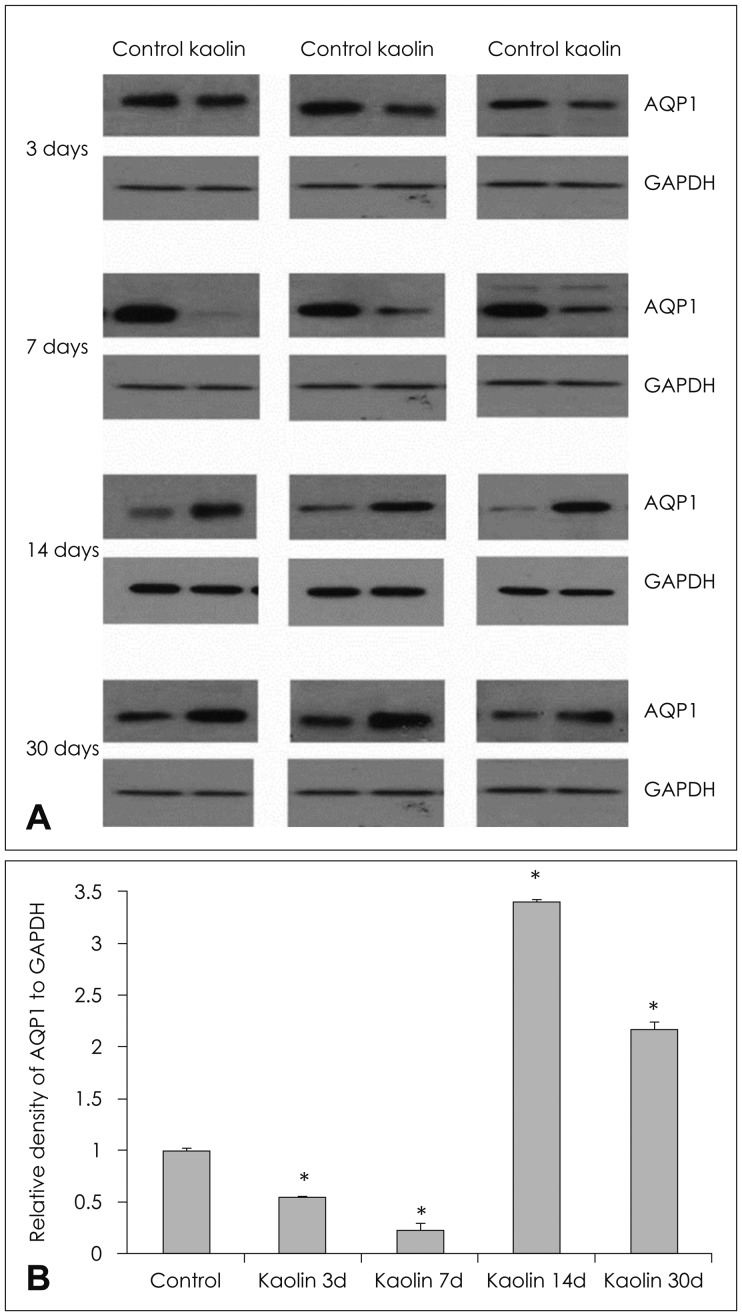
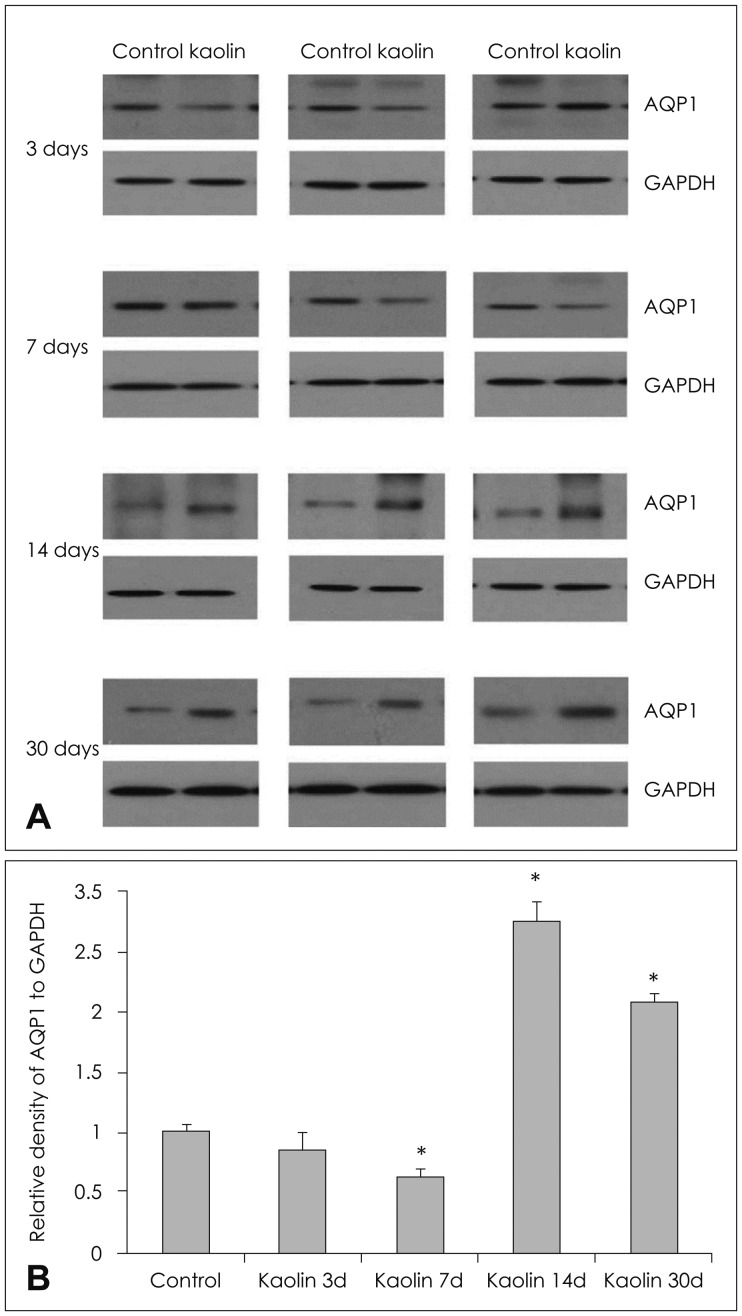
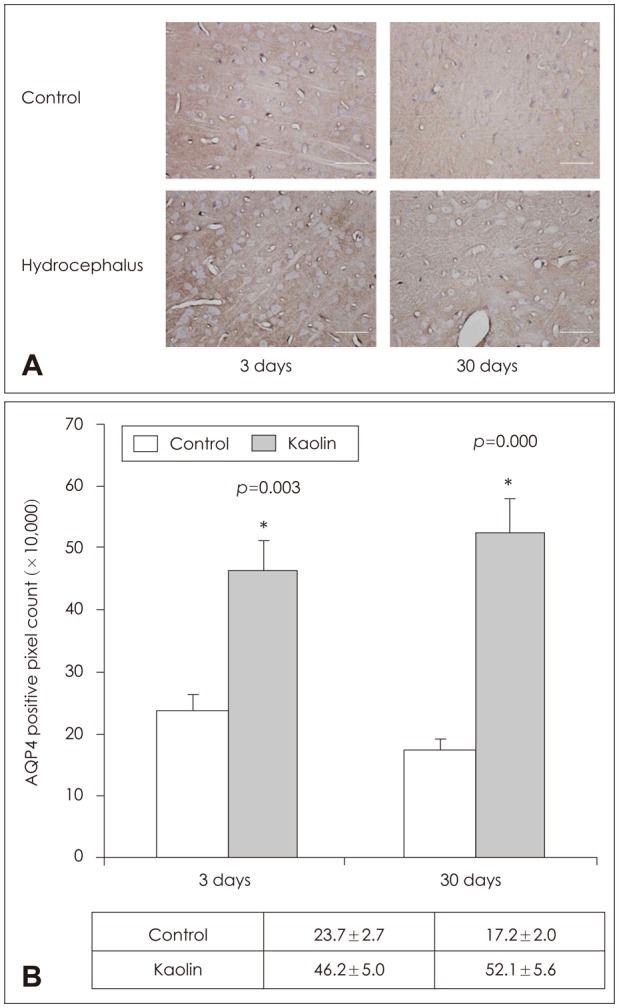
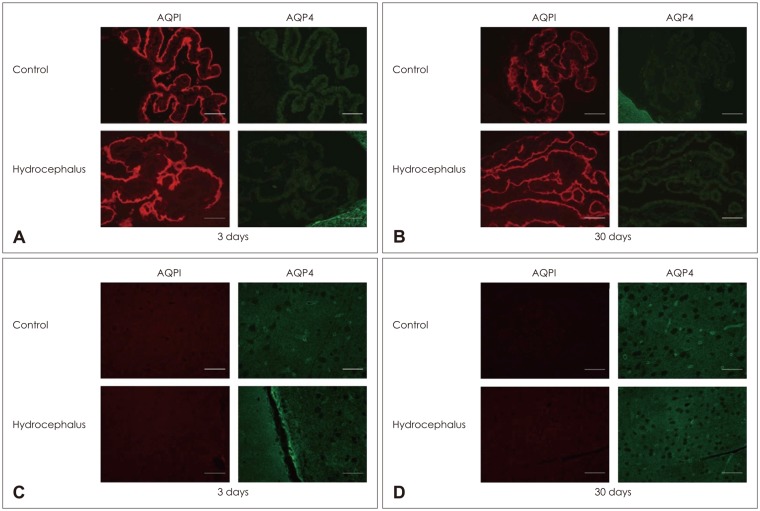




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download