This article has been
cited by other articles in ScienceCentral.
Abstract
Syringomyelia associated with tuberculous meningitis is an extremely rare condition. Only a few studies have reported clinical experience with syringomyelia as a late complication of tuberculous meningitis. Twenty-six years after a tuberculous meningitis episode, a 44-year-old man presented with progressively worsening spastic paresis of the lower limbs and impaired urinary function for 2 years. Radiological examination revealed syringomyelia extending from the level of C2 to T9 and arachnoiditis with atrophy of the spinal cord between C2 and T3. We performed laminectomy from C7 to T1, dissected the arachnoid adhesion and placed a syringo-pleural shunt via keyhole myelotomy. One year after the operation, his neurological condition improved. The postoperative control magnetic resonance imaging revealed the correctly located shunt and significantly diminished syringomyelia cavities. We aim to discuss the mechanism of syrinx formation following tuberculous meningitis and to share our surgical therapeutic experience with this rare disease entity.
Go to :

Keywords: Cerebrospinal fluid shunts, Syringomyelia, Tuberculosis, meningeal
Introduction
Tuberculous meningitis is not a rare disease, as it is one of the most common extrapulmonary manifestations of tuberculosis. However, syringomyelia following tuberculous meningitis is an extremely rare condition. Very few cases of this condition have been reported previously. Previous reports described extensive adhesive arachnoiditis and multiple lobule formation; surgical attempts failed to improve the neurological status of these patients.
We report a case of syringomyelia secondary to an inflammatory arachnoiditis owing to tuberculous meningitis, which we were able to successfully treat by performing microsurgical dissection and placing a syringo-pleural shunt.
Go to :

Case Report
A 44-year-old man presented with progressively worsening spastic paresis of the lower limbs and impaired urinary function for 2 years. He had a history of pulmonary tuberculosis and tuberculous meningitis 26 years earlier; he received chemotherapy for the pulmonary tuberculosis. Except tuberculous meningitis, he did not have a medical history of any other meningitis or trauma history.
Neurological examination revealed severe weakness of both the lower limbs and movement disability of both hands. The patient showed deep tendon hyperreflexia of the knee and the ankle. The Babinski sign was positive. He was mentally alert and had normal sensory perception. Magnetic resonance imaging (MRI) of the cervicothoracic spine revealed syringomyelia extending from the level of C2 to T9, in addition to arachnoiditis with atrophy of the spinal cord between C2 and T3 (
Figure 1A-C). Brain MRI revealed a small calcification in the left frontal horn of the lateral ventricle, which may be related to scarring from the tuberculous meningitis (
Figure 2). In brain imaging study, there was no evidences of arnold-chiari malformation. Abnormal signal change that appeared as a signal void was detected in the MRI of the lower thoracic spine. Spinal angiography, which was performed to eliminate the presence of any vascular anomalies, was unremarkable (
Figure 3).
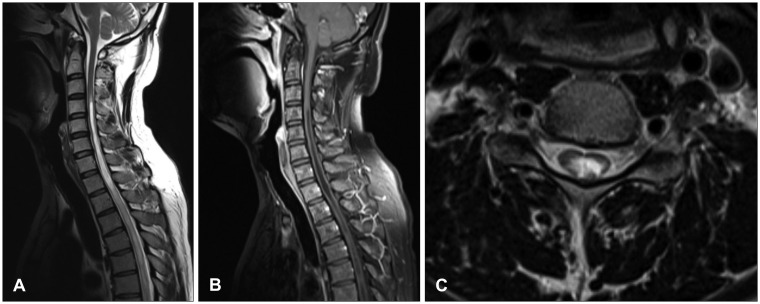 | FIGURE 1Initial magnetic resonance imaging demonstrated syringomyelia from C2 to T3 which was thought to be secondary to adhesive spinal arachnoiditis. (A) Sagittal T2 image. (B) Sagittal T1 image with contrast enhancement. (C) Axial T1 image with contrast enhancement.
|
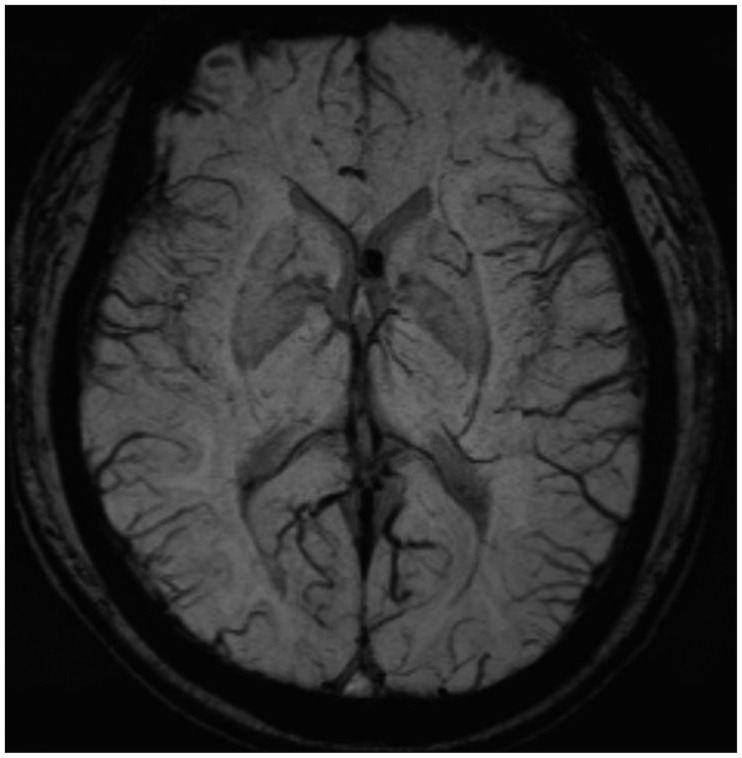 | FIGURE 2Small calcification, which may be related with tuberculosis scarring in left frontal horn of lateral ventricle was detected on brain magnetic resonance imaging.
|
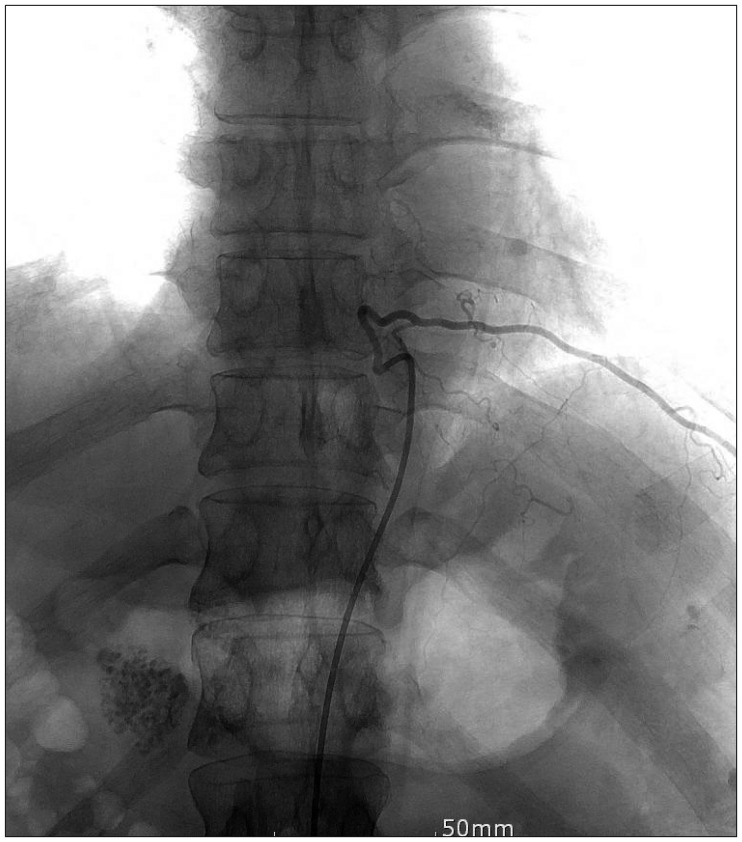 | FIGURE 3Spinal angiography was performed to rule out vascular anomaly.
|
We performed laminectomy from the C7 to the T1. After opening the dura, a thickened arachnoid was detected (
Figure 4A). The arachnoid adhesion was gently dissected under the microscope and the spinal cord was untethered. Finally, we performed keyhole myelotomy and inserted the proximal end of a syringo-pleural shunt via the keyhole and secured the catheter with dura (
Figure 4B). The distal end of the syringo-pleural shunt was placed in the left pleural cavity between the fourth and fifth ribs.
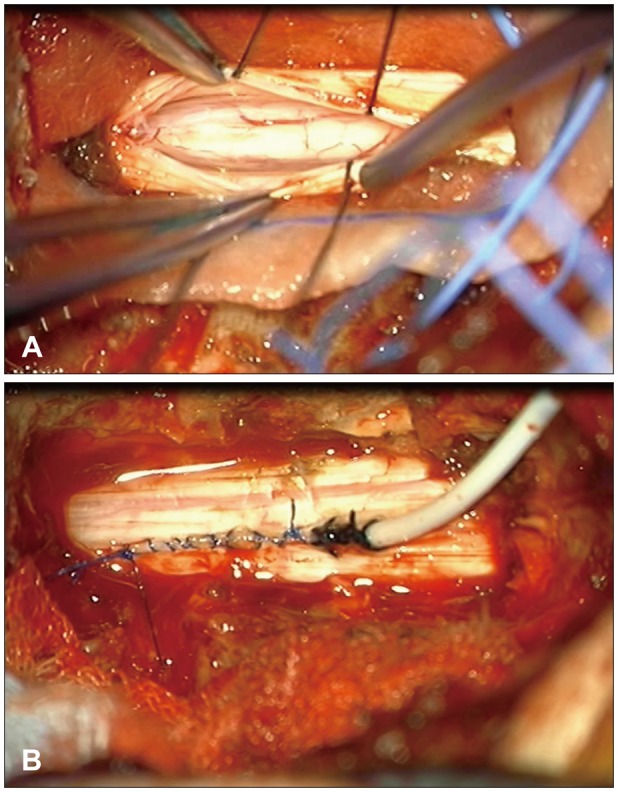 | FIGURE 4On microscopic operative view. (A) Photograph shows thickened arachnoid and dura mater. (B) Catheter tip was placed in the syrinx cavity and secured with dura.
|
Post-surgery, the weakness in the legs improved and the spasticity of the lower extremities decreased. The postoperative MRI confirmed the diminished size of the syrinx and decreased cerebrospinal fluid (CSF) accumulation in the syringeal cavity.
One year post-surgery, the patient's gait improved; hence, he was capable of walking without assistance. The weakness and spasticity of the lower extremities further improved. The follow-up MRI revealed that the syringomyelia cavities were significantly diminished in size and the proximal end of the shunt was accurately inserted (
Figure 5A and B).
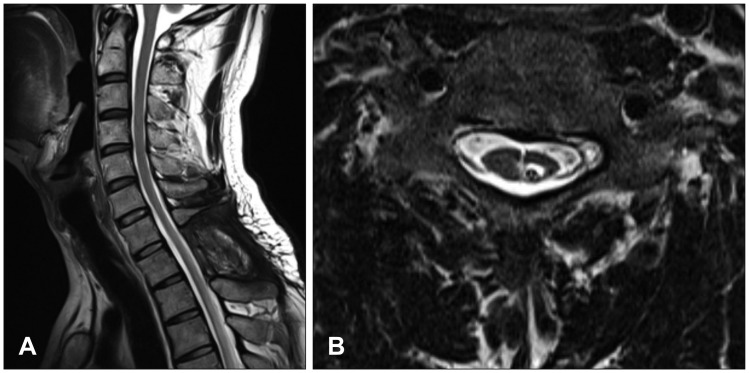 | FIGURE 5One year after operation, magnetic resonance imaging demonstrated catheter positioned properly in syrinx and diminished syringomyelia. (A) Sagittal T2 image. (B) Axial T2 image.
|
Go to :

Discussion
Several possible mechanisms of meningitis-induced syrinx formation have been reported. First, a focal scar caused by inflammatory arachnoiditis could lead to the formation of a syrinx. A focal scar could disturb the circulation of CSF in the spinal cord, thereby causing CSF congestion in the central canal of the spinal cord in the Virchow-Robin spaces. This focal cystic dilation could progressively deteriorate by the obstruction of the Virchow-Robin spaces, eventually forming a syrinx.
5)
Other possible mechanisms have also been proposed. Caplan et al.
2) proposed that obliterative endarteritis secondary to the occlusion of spinal cord vessels could lead to ischemic damage. Syrinx formation could result from the process of recovery of the injury and the spinal cord. Chang and Nakagawa
3) proposed a novel theory for the pathogenesis of syringomyelia associated with spinal adhesive arachnoiditis. According to their theory, the pulsatile CSF pressure forces the pressure inside the spinal cord to become significantly higher than that outside the spinal cord. Thus, the repetitive formation of a pressure gradient at each CSF pulse leads to the leakage of CSF from the central canal, causing interstitial edema and eventually, syrinx formation.
In our cases, we estimated the formation of syringomyelia as a sequela of tuberculous meningitis. First, the patient did not have any other predominant medical episodes which lead to syringomyelia of the spinal cord. He did not have trauma history and any other meningitis and congenital malformation such as arnold-chiari syndrome. And we could confirm the thickened arachnoid in the operative field finding and small calcification lesion in left ventricle was probably the sequelae of tuberculous meningitis.
Tuberculous meningitis is not a rare disease, the incidence of syringomyelia secondary to tuberculous meningitis is extremely low. Previous studies have suggested that it is caused by an increase in the degree of inflammation. Syrinx formation is associated with the degree of arachnoiditis and the patency of the perivascular space. If the inflammation in the lesion is minimal, then the scarring would be minimal and the compliance of the spinal subarachnoid space would not be extended, thereby preventing syrinx formation.
6) If the inflammation in the tuberculous meningitis is severe, syringomyelia occurs under specific conditions such as tuberculous endarteritis producing softening of the spinal cord parenchyma, scarring in the spinal subarachnoid space severe enough to reduce the compliance of the spinal subarachnoid compartment and patent perivascular spaces in the spinal cord providing a conduit for the CSF to enter the parenchyma.
11)
A classic treatment of syringomyelia is direct drainage of the cyst in patients who show symptoms.
13) Although direct treatment approaches such as myelotomy have been suggested, such interventions may lead to deterioration in the postoperative neurological conditions owing to direct intervention in the spinal cord and the rate of recurrence after procedures would be very high. Another treatment option for syringomyelia is adhesive arachnoid dissection and an additional duraplasty. Klekamp
8) reported achievement of clinical stability by arachnoid dissection and decompression with duraplasty in 83% of patients with focal arachnoiditis extending <2 vertebral levels. Edgar and Quail
4) performed an arachnoid dissection followed by a generous dural graft consisting mainly of fascia lata, anchored laterally to the paraspinal tissues. Ohata et al.
12) described the case of a 47-year-old man presented with syringomyelia following tuberculous meningitis. They performed microsurgical adhesiolysis and expansive duraplasty and noted a postoperative shrinkage of the syrinx cavity.
12) However, duraplasty in operations involving the spinal cord can easily create a leakage point in the dura, which may increase the risk of postoperative CSF leakage.
Placing a syringo-pleural or syringo-subarachnoid shunt is an alternative surgical treatment option for syringomyelia associated with spinal adhesive arachnoiditis.
8) Cacciola et al.
1) reported favorable clinical outcomes in 20 patients with syringomyelia refractory to restoration of CSF flow. On the contrary, Koyanagi et al.
10) reported their therapeutic experience with syringo-pleural shunts against syringomyelia following spinal arachnoiditis. Despite procedures, neurological improvement was recognized in only 60% of the patients. They recommended decompressive operation of the subarachnoid space of the spinal cord as the primary treatment option in such cases.
Recent study revealed a dissatisfactory long-term prognosis of recurrence rate of the syrinx. Klekamp et al.
9) reported that shunting of the syrinx was associated with recurrence rates of 92% and 100% for focal and extensive scarring, respectively. According to their study, successful long-term clinical outcomes required microsurgical dissection of the arachnoid scar and decompression of the subarachnoid space with a fascia lata graft. Similarly, Kaynar et al.
7) suggested multiple location formation of syrinx and extensive arachnoiditis as possible causes of failure of syrinx shunting.
In our study, we considered several operative techniques that have been suggested to treat syringomyelia. The extent of syringomyelia was relatively large, as it extended from C2 to T9. However, the preoperative MRI did not reveal a focal obstruction or multiple lobule formation and wide laminectomy of cervicothoracic spine would aggravate the kyphotic deformity, postoperatively. Thus, we did not consider extensive multi-level laminoplasty or laminectomy. Instead, we planned to restore the compliance of the spinal subarachnoid space by dissecting the arachnoid adhesion and diverting the CSF flow by placing a syringo-pleural shunt at the level of C7 T1 spine. The reason to select the operative level is that the level is the center of the syrinx formation and syrinx formation was well dilated and developed in the C7 T1 spine level.
Go to :

Conclusion
A syringo-pleural shunt can be used for the management of syringomyelia associated with adhesive arachnoiditis after microsurgical dissection of the arachnoid adhesion for a more satisfactory long-term clinical outcome.
Go to :

Acknowledgement
This work was supported by a 2-year research grant form Pusan National University.
Go to :

Notes
Go to :

References
1. Cacciola F, Capozza M, Perrini P, Benedetto N, Di Lorenzo N. Syringopleural shunt as a rescue procedure in patients with syringomyelia refractory to restoration of cerebrospinal fluid flow. Neurosurgery. 2009; 65:471–476. PMID:
19687691.

2. Caplan LR, Norohna AB, Amico LL. Syringomyelia and arachnoiditis. J Neurol Neurosurg Psychiatry. 1990; 53:106–113. PMID:
2313296.

3. Chang HS, Nakagawa H. Theoretical analysis of the pathophysiology of syringomyelia associated with adhesive arachnoiditis. J Neurol Neurosurg Psychiatry. 2004; 75:754–757. PMID:
15090573.

4. Edgar R, Quail P. Progressive post-traumatic cystic and non-cystic myelopathy. Br J Neurosurg. 1994; 8:7–22. PMID:
8011197.

5. Fehlings MG, Bernstein M. Syringomyelia as a complication of tuberculous meningitis. Can J Neurol Sci. 1992; 19:84–87. PMID:
1562914.

6. Fischbein NJ, Dillon WP, Cobbs C, Weinstein PR. The “presyrinx” state: a reversible myelopathic condition that may precede syringomyelia. AJNR Am J Neuroradiol. 1999; 20:7–20. PMID:
9974051.
7. Kaynar MY, Koçer N, Gençosmanoğlu BE, Hanci M. Syringomyelia-as a late complication of tuberculous meningitis. Acta Neurochir (Wien). 2000; 142:935–938. discussion 938-939. PMID:
11086834.
8. Klekamp J. Treatment of syringomyelia related to nontraumatic arachnoid pathologies of the spinal canal. Neurosurgery. 2013; 72:376–389. PMID:
23208064.

9. Klekamp J, Batzdorf U, Samii M, Bothe HW. Treatment of syringomyelia associated with arachnoid scarring caused by arachnoiditis or trauma. J Neurosurg. 1997; 86:233–240. PMID:
9010425.

10. Koyanagi I, Iwasaki Y, Hida K, Houkin K. Clinical features and pathomechanisms of syringomyelia associated with spinal arachnoiditis. Surg Neurol. 2005; 63:350–355. discussion 355-356. PMID:
15808720.

11. Muthukumar N, Sureshkumar V. Concurrent syringomyelia and intradural extramedullary tuberculoma as late complications of tuberculous meningitis. J Clin Neurosci. 2007; 14:1225–1230. PMID:
18029276.

12. Ohata K, Gotoh T, Matsusaka Y, Morino M, Tsuyuguchi N, Sheikh B, et al. Surgical management of syringomyelia associated with spinal adhesive arachnoiditis. J Clin Neurosci. 2001; 8:40–42.

13. Williams B, Page N. Surgical treatment of syringomyelia with syringopleural shunting. Br J Neurosurg. 1987; 1:63–80. PMID:
3267280.

Go to :






 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download