This article has been
cited by other articles in ScienceCentral.
Abstract
Thoracolumbar paraspinal myonecrosis can be developed with various etiologies. It can induce compartment syndrome of spinal muscles and cause elevated pressure on back muscles, resulting in severe back pain. Thoracolumbar paraspinal myonecrosis is a very rare disease. There are only a few studies about paraspinal myonecrosis. Here we report a case of a spontaneous thoracolumbar paraspinal myonecrosis in a patient who had asymptomatic abdominal aortic dissection. Through this case, etiologies, clinical features, radiologic findings, and treatment options for thoracolumbar paraspinal myonecrosis are discussed.
Go to :

Keywords: Aortic dissection, Back pain, Myonecrosis
Introduction
Thoracolumbar paraspinal myonecrosis can develop with various etiologies. It can cause compartment syndrome of spinal muscles and lead to elevated pressure on back muscles, resulting in severe back pain. Thoracolumbar paraspinal myonecrosis is a very rare disease. Here we report a case of spontaneous thoracolumbar paraspinal myonecrosis in a patient who had asymptomatic abdominal aortic dissection. Through this case, etiologies, clinical features, radiologic findings, and treatment options for thoracolumbar paraspinal myonecrosis are discussed.
Go to :

Case Report
A 47-year-old male patient visited the outpatient clinic with severe back pain. The patient underwent ascending aorta graft replacement and aortic valve replacement at our Thoracic Surgery Department due to aortic dissection a year ago. The patient had asymptomatic abdominal aortic dissection. He had been treated with anti-coagulant agents. His back pain started five days prior to the outpatient clinic visit. His visual analog scale (VAS) score was 8. Blood pressure was 120/80 mmHg. Heart rate was 89 beats/min. Body temperature was 36.7℃. Direct tenderness, skin redness, and swelling of thoracolumbar junction of the back were observed in physical exam. Laboratory test results showed white blood cell (WBC) count of 9010×10
9/L, hemoglobin (Hb) level of 12.5 g/dL, C-reactive protein (CRP) level of 1.15 mg/dL, and erythrocyte sedimentation rate of 40 mm. Thoracolumbar spine magnetic resonance imaging (MRI) revealed abdominal aortic dissection and swelling of left multifidus and longissimus. The MRI also showed diffuse enhancement in the left psoas muscle and longissimus with focal low signal from T11 to L4 level. However, the MRI did not show any other cause of back pain (
Figure 1). We decided to treat the patient conservatively. After bed rest and non-steroidal anti-inflammatory drugs (NSAIDs) medication for two weeks, the patient's pain was worsened based on VAS score (8–9). His muscle tenderness and swelling were aggravated. Laboratory tests revealed WBC count of 10010×10
9/L, Hb level of 12.0 g/dL, and CRP level of 3.1 mg/dL. Therefore, we performed biopsy and tissue culture of the muscle. The biopsy was performed at the operation room under local anesthesia. A 3-cm linear skin incision on T12 level was made. The longissimus fascia was incised. Using pituitary forceps, necrotic muscle was biopsied. Abnormal tissue was captured as much as possible. It was sent to pathologists. After debridement, skin suture was done with fascia open. The patient underwent bed rest for 1 day. A week later, the pain was mostly relieved. The patient was discharged. The pathologic finding was muscle necrosis. There was no bacterial growth based on culture study. Six months later, the patient did not complain of back pain or any other symptoms.
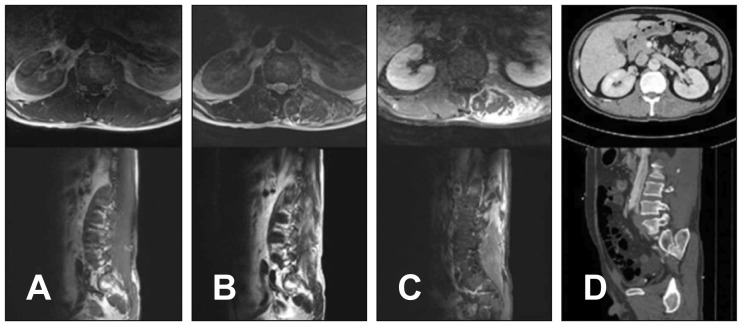 | FIGURE 1Thoracolumbar spine magnetic resonance imaging of the patient in the case report. (A) Ill-defined isointense to muscle mass in T1 image, (B) heterogeneously peripheral hyperintense and central hypointense in T2 image, (C) heterogeneously peripheral enhancement lesion in contrast enhance view, and (D) muscle enlargement with decreased attenuation in computed tomography image in the left posterior paraspinal muscle and left psoas muscle from T11 to L4 level.
|
Go to :

Discussion
Myonecrosis is a rare condition. Thoracolumbar paraspinal myonecrosis is extremely rare.
39) Typical clinical symptoms include severe back pain, swelling, and tenderness. Pain seems to be developed by tissue swelling due to muscle necrosis which can lead to increased pressure within the muscle fascia.
8) Myonecrosis has many causes. It can be divided into infectious necrosis and ischemic necrosis. Bacteria such as
Clostridium,
Bacilus,
Aeromonas hydrophilia, and
Stapylococcus can cause infectious necrosis.
17) However, the present case was ischemic myonecrosis. Other causes include sickle cell disease, trauma, intra-arterial chemotherapy, and rhabdomyolysis.
59) The patient of this case had asymptomatic abdominal aortic dissection. The vascular anatomy of lumbar spine is shown in
Figure 2.
23) Dorsal segmental artery from abdominal aorta supplies lumbar spinal muscle. Osamura et al.
9) have presented a case of thoracolumbar paraspinal myonecrosis after abdominal aorta graft surgery.
9) However, the present case was myonecrosis with non-operative asymptomatic aortic dissection. The patient of the present case had abdominal dissection from T10 to L5 level. A thrombus due to abdominal dissection might have caused an occlusion of the segmental artery dorsal branch. Myonecrosis might occur due to thrombus with aortic dissection.
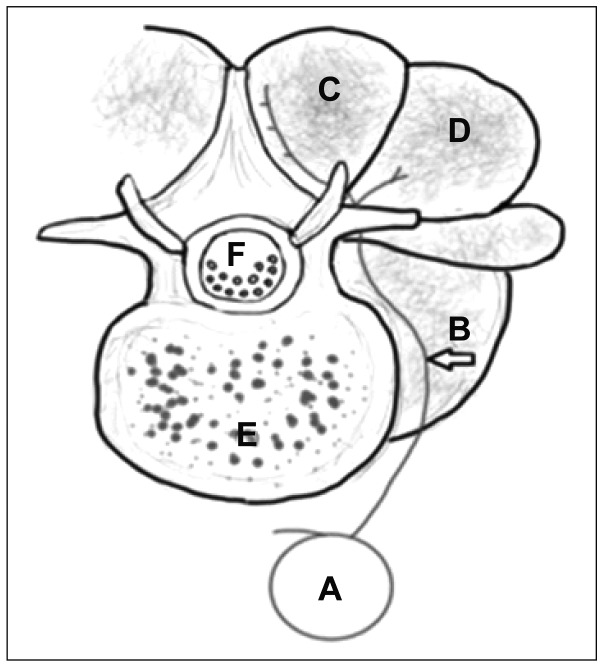 | FIGURE 2Anatomic drawing of axial view of lumbar spine (A) aorta, (B) dorsal segmental artery, (C) multifidus, (D) longissimus, (E) vertebral body, and (F) thecal sac.
|
Imaging studies are important for diagnosing myonecrosis. Computed tomography (CT) can reveal extension and location of lesion or affected muscle. MRI is the gold standard to diagnose myonecrosis.
9) Characteristic features include loss of intermuscular septum in T1-weighted image, diffuse enlargement of the lesion muscle, and subcutaneous edema in T2 fat suppression. A diffuse enhancement can be seen in T1-enhanced image. Focal low signal intensity of muscle necrosis can be observed.
456)
Thoracolumbar paraspinal myonecrosis should be distinguished from cellulitis, hematoma, abscess, pyomyositis, fasciitis, and malignancy. Myonecrosis differs from diseases mentioned above based on laboratory data and history. However, a biopsy is needed for confirmation. Treatment of common myonecrosis is possible with conservative therapy of NSAIDs medication without compartment syndrome.
5) However, when compartment syndrome occurs and pressure on the muscle increases, surgical treatment should be considered. Common treatment of muscle compartment syndrome is fasciotomy.
8) In this case, biopsy can be a diagnostic tool and fasciotomy can be done at the same time to reduce pressure inside the muscle. In the present case, the dramatic improvement in the patient's back pain seemed to be due to pressure drop in the muscle as fasciotomy was performed. For patients with risk factors such as aortic dissection, ischemic myonecrosis should be considered. In particular, myonecrosis should be considered for patients with abdominal aorta lesions and back pain. It is important to perform an accurate diagnosis and fast fasciotomy if compartment syndrome is suspected. Accurate diagnosis and fasciotomy can be considered as a way to prevent further myonecrosis and control back pain.
Go to :

Conclusion
It is difficult to make a rapid diagnosis due to the rarity of thoracolumbar paraspinal myonecrosis. Therefore, when a patient has a risk factor such as abdominal aortic dissection and back pain, tenderness, and muscle swelling, myonecrosis should be suspected. It is important to make an accurate imaging study and make a differential diagnosis with infection and malignancy. Fasciotomy and biopsy are necessary for accurate diagnosis and pain relief.
Go to :






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download