This article has been
cited by other articles in ScienceCentral.
Abstract
Pneumocephalus is commonly seen after craniofacial injury. The pathogenesis of pneumocephalus has been debated as to whether it was caused by ball valve effect or combined episodic increased pressure within the nasopharynx on coughing. Discontinuous exchange of air and cerebrospinal fluid due to “inverted bottle” effect is assumed to be the cause of it. Delayed tension pneumocephalus is not common, but it requires an active management in order to prevent serious complication. We represent a clinical case of a 57-year-old male patient who fell down from 3 m height, complicated by tension pneumocephalus on 5 months after trauma. We recommend a surgical intervention, but the patient did not want that so we observe the patient. The patient was underwent seizure and meningitis after 7 months after trauma, he came on emergency room on stupor mentality. Tension pneumocephalus may result in a neurologic disturbance due to continued air entrainment and it significantly the likelihood of intracranial infection caused by continued open channel. Tension pneumocephalus threat a life, so need a neurosurgical emergency surgical intervention.
Go to :

Keywords: Cerebrospinal fluid leak, Head injury, Tension pneumocephalus
Introduction
Pneumocephalus is a commonly observed complication of craniofacial injury. It is mostly found in 20% to 30% of patients with traumatic cerebrospinal fluid (CSF) fistula, and can also occur in the patients who have no CSF rhinorrhea. Additionally, it can be caused by infection of the sinuses, including the ear canals, and can occur after cranial surgeries, such as skull base tumor surgery.
2513) A small volume (1–2 cc) of pneumocephalus is often observed after head injury and is naturally resolved without the need for special treatment. However, persistent fistula is likely to occur in the case of a large volume of air, and causes “succession splash” upon movement of the head.
Pneumocephalus is assumed to be caused by discontinuous exchange of air and CSF due to the “inverted bottle” effect when the intracranial pressure (ICP) is lower than the atmospheric pressure. However, there is ongoing debate on whether the ball valve effect or combined episodic increased pressure within the nasopharynx during coughing may be involved.
11121316)
Although tension pneumocephalus is not common, it requires active management to prevent serious complications.
7)
Go to :

Case Report
A male patient, 57 years old, was admitted to the hospital for treatment, following a fall from a height of 3 m on September 24, 2016. The imaging test performed at the time of admittance showed several injuries, including skull fracture frontotemporal bone, and epidural hematoma, as well as a bilateral blowout zygoma fracture. However, the images did not show any specific lesions which required invasive management from a neurosurgical perspective; therefore, the patient's condition was monitored over time. On the 10th day after hospitalizing, the patient underwent open reduction using a Medpor sheet (Stryker, Kalamazoo, MI, USA) for the tripod zygoma blowout fracture, right (Rt; impure type) and left (Lt; medial wall fracture) blowout fracture, and maxilla fracture Lt (nasofrontal buttress fracture) at the plastic surgery unit before being discharged from the hospital without any complications. Throughout the period of hospitalization, no signs of CSF leakage were observed. The patient also showed no signs of fever and lacked any sign of inflammation perioperatively, with white blood cell (WBC) count, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) being in the normal range.
Five months after the initial trauma (March 8, 2017), the patient was admitted to the emergency room with left side hemiparesis (motor grade IV) that had commenced three days previously. This was accompanied by symptoms such as dysarthria, headache, and dizziness; however, the patient's mental status was alert, his Glasgow Coma Scale was E4V5M6, and his mental functions were noted to be intact.
Pneumocephalus was observed at the Rt frontal area in the computed tomography scan which was performed after visiting the hospital (
Figure 1). As tension pneumocephalus with mass effect was suspected, the patient was hospitalized and the condition monitored over time. We recommended that the patient receive surgical treatment; however, the patient was content with further monitoring as his condition appeared to be gradually improving, despite some fluctuations in his symptoms. Therefore, we underwent conservative care and followed up over time with respect to the patient's option. There was no considerable change observed in the intracranial air volume from the follow-up test conducted during the hospitalization period, nor any sign of CSF leakage (
Figure 2).
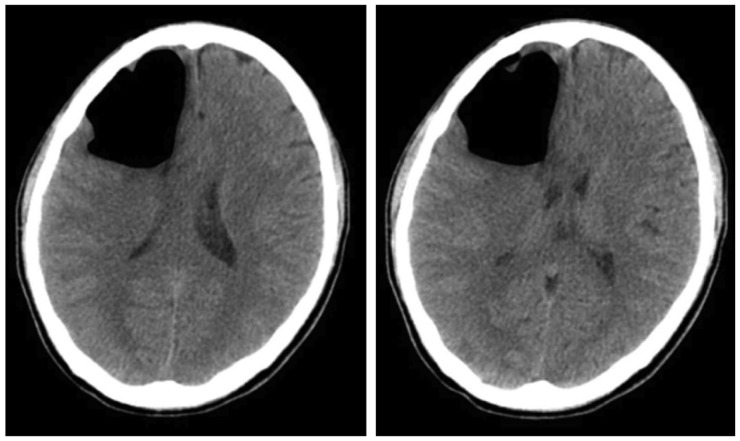 | FIGURE 1Newly developed a large amount of pneumocephali at the right frontal lobe with mild mass effect, compressing the right lateral ventricle and shift in the mid-line on brain computed tomography.
|
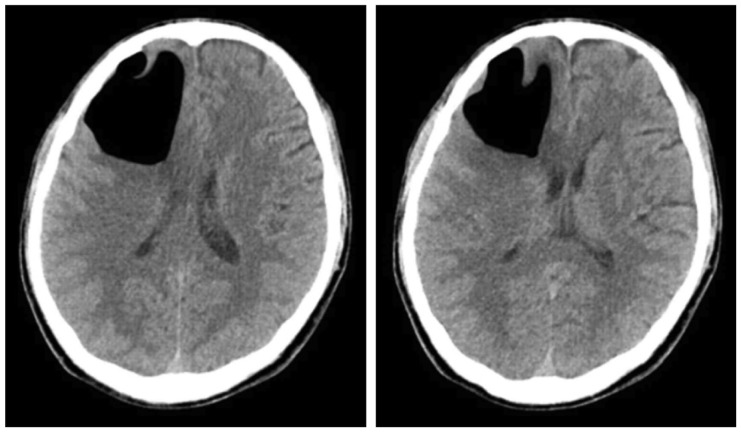 | FIGURE 2This is the last follow-up computed tomography on second admission period; again noted, a large amount of pneumocephali at the right frontal lobe. Mild mass effect, compressing the right lateral ventricle and shift in the mid-line. Little interval changes in the large pneumocephali at the right frontal lobe.
|
Following this period, the patient did not experience any specific symptoms while undergoing outpatient evaluation. Several weeks later, an image follow-up was conducted at the emergency room upon discovery of a worsening headache starting from May 12 or 13, 2017, at seven months since injury (
Figure 3). He was discharged from the hospital in the absence of a change in Rt frontal pneumocephalus, but was re-admitted to the emergency room for a generalized tonic-clonic (GTC)-type seizure on May 14, 2017. Several tests were conducted, showing that the pneumocephalus in the Rt frontal area had reduced and the brain parenchyma had expanded, accompanied by fluid collection; signs of ventriculitis were also observed (
Figure 4 and
5). Thus, the patient was hospitalized and is now under conservative care on a course of antibiotics. Mentally, he is in a state of constant stupor, and it is unlikely that his symptoms will improve following course completion. The electroencephalography taken at the time showed generalized continuous slow wave, indicating moderate to severe cerebral dysfunction. Laboratory findings showed signs of infection (WBC 23.20 10
9/L, ESR 8 mm/h, CRP 57.66 mg/L); the CSF tap showed the following results: WBC 2,400/µL, red blood cell (RBC) 20/µL, protein 1,099.2 mg/dL, glucose <10 mg/dL, and
Streptococcus Pneumoniae was detected in the CSF culture. The sudden infection that developed despite the lack of signs of postoperative inflammation and, particularly, the lack of a history of infection was most likely caused by tension pneumocephalus rather than any other cause.
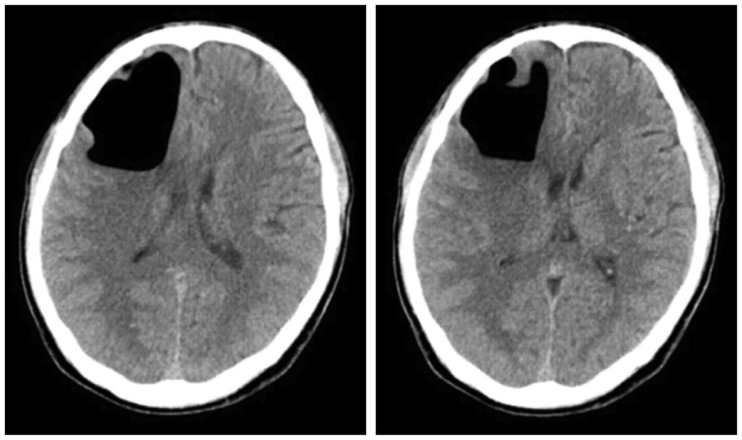 | FIGURE 3Little interval changes in the large pneumocephali at the right frontal lobe on brain computed tomography.
|
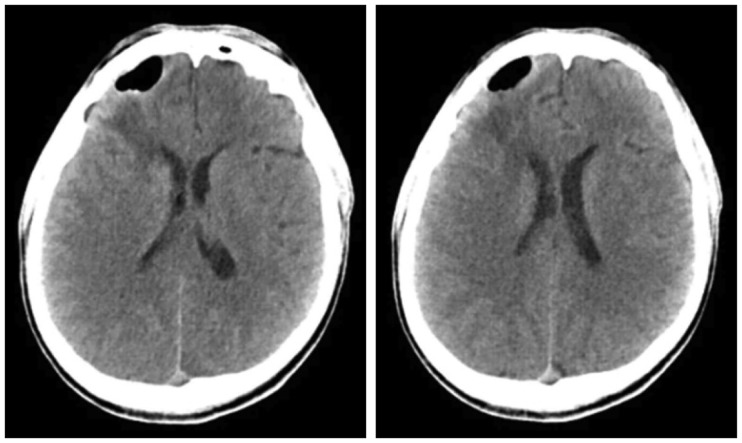 | FIGURE 4Intervally marked decreased intraparenchymal pneumocephalus in right frontal lobe and increased heterogeneous density fluid collection and perilesional low density edema in the cavity of right frontal lobe on brain computed tomography.
|
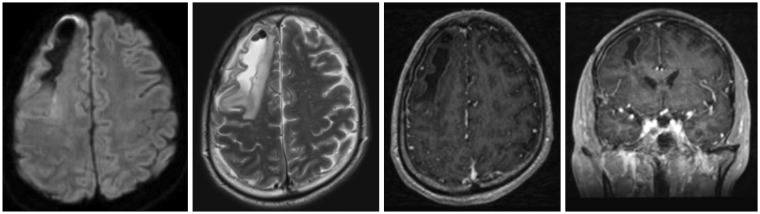 | FIGURE 5About 6.5×1.5 cm sized irregular intraparenchymal fluid collection with air bubbles and perilesional edema in right frontal lobe with mild enhancement along the wall of fluid collection was observed on brain magnetic resonance imaging (MRI) of axial views of the diffusion-weighted, the T2-weighted, the dynamic contrast enhanced of a coronal image, the dynamic contrast enhanced image along the clockwise direction from a left upside figure. It is mean rule out chronic hematoma, differential diagnosis combined early inflammatory change. Also, decreased pneumocephalus was observed on brain MRI enhance.
|
Go to :

Discussion
Pneumocephalus, that is, an air collection in the skull and brain parenchyma, should not occur under normal circumstances.
89) Generally, it is caused by complications occurring because of head injuries and is found in 3.9% to 9.7% of patients with head injuries, according to one journal
10); it is said to occur in 100 % of cases following craniotomy.
14)
When an air accumulation occurs in the skull within 72 hours of injury, it is termed “acute,” whereas it is termed “delayed” after 72 hours.
1) Most patients experience no symptoms and, in these occurrences, the accumulation is known to be benign. In rare cases, air cavity is causative of the mass effect and can trigger neurological deterioration-this is known as tension pneumocephalus.
Particularly, delayed tension pneumocephalus is a rare phenomenon, with many reports in journals emphasizing the requirement for urgent surgical treatment. We present our case in an attempt to stress the importance of surgical treatment for pneumocephalus, as the patient in this case initially showed improvement of symptoms in response to conservative care but later experienced worsening of symptoms.
There are two theories regarding the mechanism of pneumocephalus, one of which is dandy theory of ball valve and the other is Horowitz “inverted soda bottle effect”.
46) The former theory suggests that air is entrained in one direction and creates an air trap. The latter states that pneumocephalus occurs because of the buildup of negative ICP as a result of extensive CSF loss or Valsalva, caused by low ICP.
Clinical presentation of tension pneumocephalus can include symptoms such as headache, seizure, agitation, delirium, and reflex abnormality etc. which can cause alteration of mental status in severe cases. In situations in which tension pneumocephalus commonly occurs, the frontal lobe syndrome may also develop.
8)
Pneumocephalus is a lesion that commonly occurs in patients with head trauma. Most of the cases can be cured spontaneously, but can cause neurological manifestation in rare instances. In particular, tension pneumocephalus may result in neurological disturbance because of continued air entrainment and may significantly increases the likelihood of intracranial infection because of the presence of a constantly open channel.
In terms of treatments, it is possible to directly remove the air, and control the infection via repair of the skull base alongside a course of antibiotics. Conservative care is generally the initial treatment option, but in the case of tension pneumocephalus there are many situations in which urgent treatment is required.
715)
The patient in this case study was again admitted to hospital due to worsening symptoms accompanied by seizures and a change in mental state. Several tests were conducted and detected infection as well as no improvement of consciousness.
When clinical signs appear, such as intracranial hypertension or impaired consciousness, that endanger the life of the patient, treatment consists of emergent decompression to alleviate pressure on the brain parenchyma.
3)
On the basis of the patient's clinical symptoms at the time of the hospital visit, we should have recommended surgical treatment to the patient and his family more aggressively, emphasizing on the importance of direct air removal.
Go to :

Conclusion
In rare cases, there is a chance that patients could develop delayed pneumocephalus; thus, neurosurgeons should always take this into consideration during regular follow-up visits. Further, it is necessary to actively recommend surgical treatment to patients who develop pneumocephalus.
Go to :










 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download