Introduction
Brain death was originally defined as the complete loss of brain function in a Harvard Medical School publication from 1968.
1) In the same year, detailed criteria of brain death were presented at the 22nd World Medical Association meeting, at which time brain death was officially recognized as death from a medical perspective.
7) A rare spinal reflex related to death of the brainstem or entire brain was reported for the first time in 1973 by Ivan
4). In 1984, Ropper
8) reported a case of a brain-dead patient who demonstrated a complex reflex movement: bilateral arm flexion to the chest, shoulder adduction, and hand crossing; he named this collective movement the Lazarus sign. In this article, we report two patients who showed a Lazarus sign after being diagnosed with brain death in order to discuss our unique treatment experiences.
Go to :

Case Report
Case 1
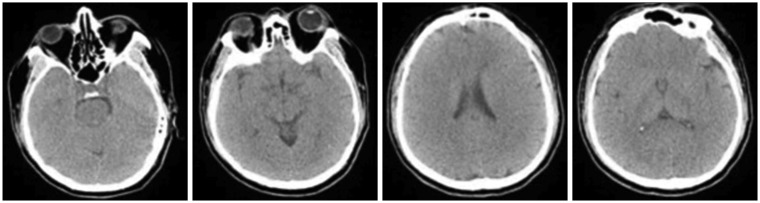
A 47-year-old woman was admitted to the hospital due to sudden severe headache. The patient presented with drowsiness initially, but soon manifested symptoms of vomiting and convulsions. She then became stuporous, with both pupils fixed at 6 mm. Brain computed tomography (CT) demonstrated subarachnoid hemorrhages in both cerebral hemispheres, a large intracerebral hemorrhage in the left Sylvian cistern area and an intraventricular hemorrhage (
Figure 1). CT angiography (CTA) was attempted to locate a possible ruptured brain aneurysm; however, the contrast media was unable to penetrate the brain due to severe brain swelling, so it was impossible to evaluate the intracranial vessels. To reduce the intracranial pressure, an emergency decompressive craniectomy was performed. In addition to the severe brain swelling, a saccular aneurysm in the left middle cerebral artery was identified that was still actively bleeding. Thus, aneurysm neck clipping was performed. The surgery lasted for 3.5 hours, with norepinephrine administered because the patient's blood pressure could not be maintained. The patient's status worsened postoperatively, and she entered a coma, with pupils fixed at 7 mm. There were no notable changes other than a slightly changing pupil size, and her vital signs remained stable. However, movement, respiration, and brainstem reflexes were all completely absent.
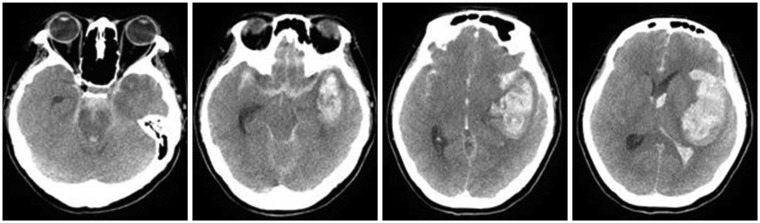 | FIGURE 1In the brain computed tomography performed on the day of admission, subarachnoid hemorrhage in both cerebral hemispheres and a large intracerebral hemorrhage in the left sylvian cistern area with intraventricular hemorrhage were found.
|
Electroencephalography (EEG) performed on postoperative day (POD) 9 to delineate brain activity showed minimal continuous, irregular, and low-amplitude slow activity, and a neurologist confirmed the results as equivalent to brain death. Subsequently, the hospital's brain death review committee evaluated the patient's status and confirmed the diagnosis. Organ donation was recommended to the family, but they declined. Limb movements not seen before were observed on POD 15; the upper limb movements included bilateral arm flexion to the chest and shoulder adduction, similar to the Lazarus sign. The hands were not completely folded together, but instead came into close proximity (e.g., praying hands) before returning to the original position. At the time of the upper limb movements, the lower limbs also showed flexion and extension. The movements occurred intermittently for 15 to 30 seconds, and were observed 3 and 4 times a day. The times of day of occurrence and the factors causing the movements were unclear. When a painful stimulus was applied, such movements were sometimes seen to occur up to 5 times; however, these movements did not always occur in response to pain. With time, their frequency decreased, and the movements disappeared around POD 30.
On POD 40, a follow-up brain CT was performed, demonstrating a large cerebral herniation at the craniectomy site and decreased corticomedullary differentiation of the cerebral hemisphere (
Figure 2), confirming the diagnosis of severe hypoxic encephalopathy. The patient remained vitally stable without any notable symptom changes until POD 219, at which time she had a drop in blood pressure and unstable vital signs; she was pronounced dead on POD 220. As her physical status continued without notable changes, we again recommended organ donation two or three times. However, the family refused organ donation stubbornly and asked to continue life-sustaining treatment, which prolonged the time until death. CT images were taken on POD 40, 65, and 142, but no other EEG tests were performed (
Figure 3 and
4). Furthermore, basic neurological tests were performed three times a day at the intensive care unit (ICU) following the brain death diagnosis, but no other changes were observed. No reflexes had been observed since POD 30.
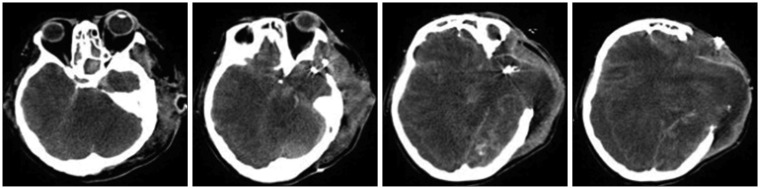 | FIGURE 2In the follow-up brain computed tomography performed on postoperative day 40, large cerebral herniation through the craniectomy site and decreased corticomedullary differentiation of the cerebral hemisphere were observed.
|
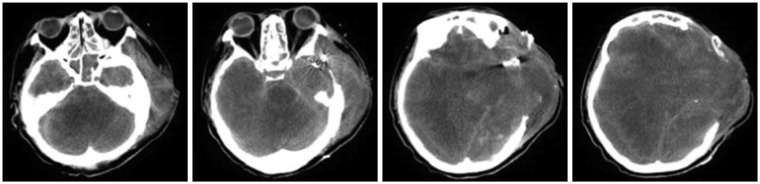 | FIGURE 3In the follow-up brain computed tomography performed on postoperative day 65, decreased cerebral herniation through the craniectomy site and no change of decreased corticomedullary differentiation of the cerebral hemisphere were observed.
|
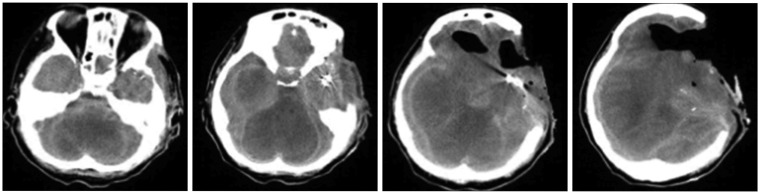 | FIGURE 4In the follow-up brain computed tomography performed on postoperative day 142, resolving intraventricular hemorrhage in left frontal area and diffuse brain swelling with severe midline shift and increased pneumocephalus in left frontal lobe were observed.
|
Case 2
A 42-year-old man was admitted to our hospital with reduced consciousness and cyanosis after getting stuck in an escalator. Immediately after he was admitted to the hospital, cardiac arrest occurred. Cardiopulmonary resuscitation was performed, and spontaneous circulation was observed 15 minutes later. He was in a coma and limb reflexes were absent; bilaterally, the pupils were fixed at 6 mm. A brain CT examination showed no evidence of intracranial injury; however, hemopneumothorax was found, and right and left tube insertions were performed. Multiple rib fractures were observed on both sides, and the patient was admitted to the thoracic surgery ICU (
Figure 5). An EEG performed on day 4 post-admission showed minimal continuous, irregular, and low-amplitude slow activities, verified as equivalent to brain death by a neurologist. His chest condition improved on day 7 post-admission, but the patient remained in a coma. Brain death was diagnosed, and he was transferred to the neurosurgery ICU for follow-up. Diffuse severe brain swelling with transtentorial herniation was found in the follow-up brain CT performed on day 9 post-admission (
Figure 6). The Brain Death Review Committee evaluated the patient's status and confirmed the diagnosis of brain death. Organ donation was recommended to the family, but they refused. Beginning on day 10 post-admission, the Lazarus sign was observed. The patient showed upper extremity movements similar to the patient in Case 1, although without spontaneous and/or reflex movements in the lower extremities. The movements occurred irregularly for 15 to 30 seconds, and were observed 1 and 2 times a day. The times of occurrence and the factors causing the movements were unclear. The movements occurred until day 20 post-admission, after which they were no longer observed. The patient remained vitally stable without any notable symptom changes over several months. As seen in Case 1, the patient's blood pressure dropped and vital signs were unstable for about a day before the patient died; he was pronounced dead on day 117 post-admission. Other than the CT image taken on POD 9, no further CT or CTA imaging was performed. A follow-up EEG was performed on POD 88, but the results continued to indicate brain death. Since the diagnosis, basic neurologic tests were performed three times a day at the ICU, and no other changes were observed.
 | FIGURE 5No notable findings were observed in the brain computed tomography performed on the day of admission.
|
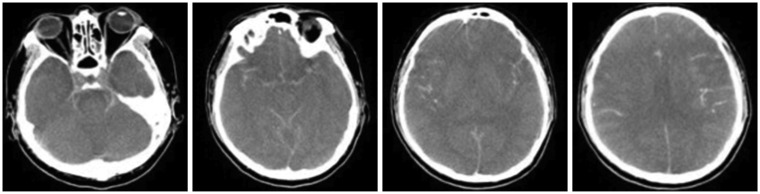 | FIGURE 6In the follow-up brain computed tomography performed on day 9 post-admission, diffuse severe brain swelling with transtentorial herniation was found.
|
Go to :

Discussion
Brain death is the state of irreversible loss of brain function due to organic brain damage. The essential criteria for establishing brain death include permanent apnea, complete unresponsiveness, and an absence of brainstem reflexes.
13) However, case studies have sparsely but continually reported that limb movements occur even in the state of brain death, with spinal reflexes being generally accepted as the likely mechanism.
458) In 1973, Ivan
4) reported that spontaneous and reflex movements were observed in 75% of brain-dead patients. A 2005 cohort study conducted by Saposnik et al.
9) with 107 brain-dead patients found that 47 patients (44%) manifested spontaneous and reflex movements. Based on these findings, it is thought that such movements occurring after brain death are not a rare phenomenon.
459)
However, the spontaneous and reflex movements described above vary widely, and one of the rarer and most complex is the Lazarus sign. Ropper's seminal paper
8) described five patients showing nonspecific spontaneous and reflex movements, including bilateral arm flexion, shoulder adduction, and raising of the arm to the neck and dropping, when the ventilator was removed or when the apnea test was performed (
Figure 7).
48) Since then, the Lazarus sign has been reported in a small number of their patients by several authors.
3912) The Lazarus sign is not common, with Saposnik et al.
9) finding it in only 2% of patients. The most accepted argument is that the Lazarus sign is caused by a spinal reflex, as with other similar movements.
812) However, it has been suggested that it is caused by a hypoxic state, in response to the early reports that the Lazarus sign occurred in a situation that induces hypoxia (e.g., during the apnea test). This hypothesis was weakened upon reports of the Lazarus sign also occurring in normoxic states.
31112) A further belief suggests that it may be caused by spinal compression or stimulation as a result of passive neck flexion or other external stimuli; however, the exact cause is yet to be elucidated.
2)
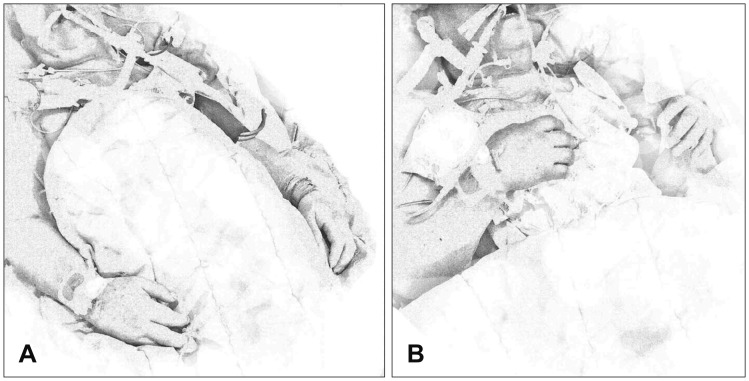 | FIGURE 7The Lazarus sign. (A) Normal posture of the brain-dead patient; (B) posture when the Lazarus sign occurs: bilateral arm flexion to the chest, shoulder adduction, and hand crossing for about 10 to 30 seconds before returning to the normal posture.
|
We find it interesting that both patients in the present study survived for over 100 days after brain death was diagnosed. It has been accepted that cardiac arrest usually occurs within 1 week after brain death.
6) However, in 1998, Shewmon
10) reported a collection of cases of brain-dead patients who survived for a long term without cardiac arrest; 175 brain-dead patients survived for over 1 week, and one of them survived for 14.5 years. Given that the two cases herein survived longer than would be expected, the question arises as to whether there is a relationship between the Lazarus sign and long-term survival after brain death. Though two cases is too small a number to address such a relationship, the hypothesis is worth investigating as it may lead to insights into both consciousness and motor function.
Recovery is impossible after brain death, and no study has reported a case in which a brain-dead patient regained consciousness. Accordingly, it may be meaningless to investigate how long a patient survives without cardiac arrest after being diagnosed as brain-dead. Further, it would also be clinically meaningless to continue life-sustaining treatment, knowing that recovery is impossible after brain death. However, it could be significant from the family's perspective. In general, when the medical staff explains brain death to the family, they report that the patient is likely to die within 1 and 2 weeks. However, if the patient survives without cardiac arrest after 1 and 2 weeks, the family may feel tremendous pain for the patient, or even false hope, in contradiction to what has been observed and described to them. The medical staff also feels helpless, because essentially only the heart still functions. This sequence of events happened in both of our cases. After the diagnosis of brain death, the families were informed that cardiac arrest would occur within 1 and 2 weeks, but more than 100 days passed without any notable changes. This period was very difficult both for the family and medical staff.
Shewmon
10) stated that age and the general health of the patient at the time of brain death affect long-term survival. If the Lazarus sign is confirmed to predict long-term survival after brain death, it would help physicians to provide families with more accurate expectations for the prognosis of brain-dead patients.
Go to :






 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download