Introduction
Massive intracranial hematomas are life-threatening emergencies that can occur after acute traumatic brain injury (TBI). Hematoma accumulation increases intracranial pressure (ICP), which can lead to brain damage and a permanent vegetative state or death. The appearance and expansion of a
de novo contralateral hematoma after surgery to reduce ICP is a rare event.
51113) On the other hand, overlooking new hematoma formation or hematoma expansion in the contralateral side can result in dismal prognoses.
17) This paper presents two clinical cases, in which transcranial sonography (TCS) was used to detect a change in contralateral hematoma volume.
Technical methods
TCS was performed by a single operator using a GE logiqE unit (GE healthcare, Milwaukee, WI, USA) and a standard abdominal convex phased-array probe with a mean central frequency of 4 MHz and an abdominal setting. A dynamic range of 45 to 50 dB was used. After applying a small amount of sterile ultrasonic gel, the probe was placed gently above the dura to avoid increasing ICP. Scanning was performed at a depth of 16 cm and the whole brain was scanned in B-mode.
Go to :

Discussion
New hematoma formation or expansion of a preexisting hematoma on the contralateral side after hematoma evacuation is unusual. The possible causes of hematoma volume increase include a ruptured meningeal arterial branch, venous lacerations causing low-tension bleeding, or a skull fracture.
817) Other factors include surgical decompression, cerebrospinal fluid fistula, aggressive anti-edema treatment, and systemic hypotension, which can all lead to intracranial hypotension.
46) The possible mechanisms responsible for the contralateral hematoma increase in Case 2 were skull fracture on the other side and reduced ICP.
In general, neurological deterioration, pupillary dilation contralateral to a hematoma, seizure, and intractably elevated ICP are clinical presentations of
de novo hematoma formation or volume expansion of a contralateral hematoma after surgery.
1417) Most neurosurgeons rely on immediate postoperative CT when hematoma formation or expansion is expected, such as, in patients with a prior contralateral skull fracture or hematoma or severe brain herniation development after ipsilateral hematoma evacuation. Although the early detection and decompression of contralateral hematoma could minimize secondary insult to the normal brain, safety concerns associated with CT scanning, surgical wound closure, and transportation for CT arise. Some authors have recommended explorative burr hole trephination on the contralateral side during first surgery,
14) but this is considered by many to be too invasive to confirm the possibility of hematoma expansion or formation. Intraoperative CT is an excellent diagnostic tool and is utilized in some institutes, but its capital cost, preparation and operating times, and the hazards posed by radiation exposure are troublesome.
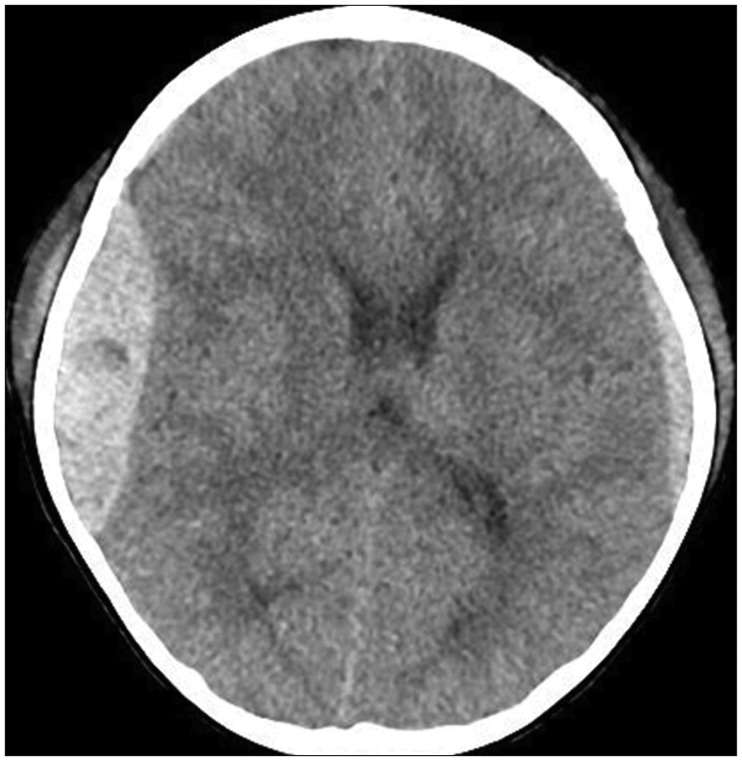
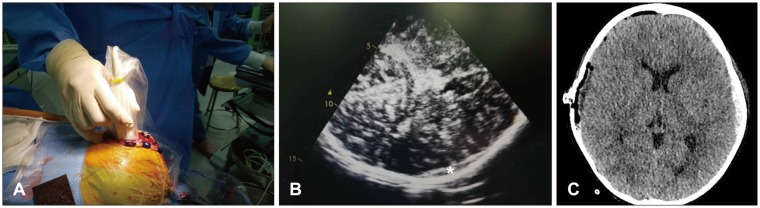
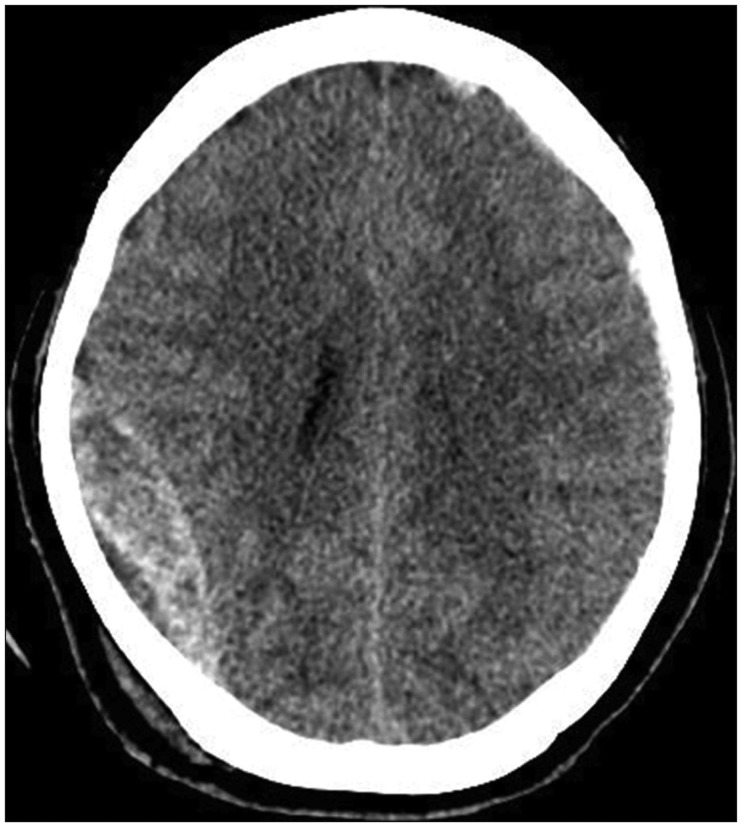
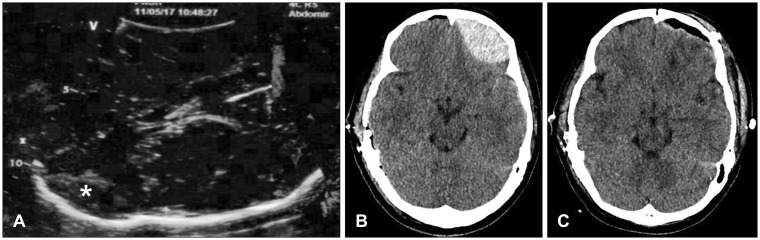
Therefore, we considered intraoperative TCS as an alternative diagnostic tool in TBI. In both described cases, we suspected the possibility of contralateral hematoma expansion because of a skull fracture and preexisting SDH on the contralateral side. In Case 1, there was no volume change in the contralateral side as determined by immediate postoperative CT. In Case 2, the hyperechoic lesion on the contralateral side was detected by intraoperative TCS (
Figure 4A). While postoperative CT was still performed to confirm the pathology, we were able to save the time required for preparation of an operation suite and anesthesiologist time for secondary operation. Had a large lesion requiring immediate evacuation been detected by intraoperative TCS, immediate postoperative CT would have been avoided and much more time been saved.
Intraoperative TCS has several advantages as a diagnostic tool. First, it can provide relatively sensitive images of the brain. Accurate descriptions of sonographic features of cerebral anatomy are available in the literature. Some reports have shown hematomas, midline shifts, and ventricular enlargements can be detected using a low-frequency probe through the temporal bone of an intact skull.
237910) Others have suggested this technique be used at bed-side in patients with decompressive craniectomy.
121516) The quality of the image is good, and the accuracy is not reduced by dural grafts. Furthermore, after bone flap removal, the frequency of the probe may be higher than that of TCS.
1) Therefore, a better definition of the superficial part of brain parenchyma can be obtained. In addition, when massive intraoperative brain herniation is encountered, intraoperative TCS can be particularly useful for differentiating various ipsilateral pathologies requiring surgery, such as, intracerebral hematoma or SDH with severe brain swelling.
Second, intraoperative TCS reduces the time and effort required for imaging as compared with postoperative CT, preparation and scanning of whole brain takes less than 10 minutes, and surgical wound closure and transport for CT are not required. Furthermore, the use of TCS reduces the risk of patient deterioration during transportation and decision making time.
Third, the equipment required for intraoperative TCS is readily available in most institutes. Ultrasonography is commonly used by anesthesiologists and several intraoperative Doppler sonography machines widely used for neurovascular surgery can be converted to B-mode ultrasonography by the simple addition of a probe. In addition, TCS poses no risk of radiation exposure to patient or health care providers.
However, intraoperative TCS also its disadvantages, the most obvious of which is that its imaging quality is limited compared with that of CT. Caricato et al.
7) described near non-visualization of hypodense ischemic lesions or late phase of hemorrhages on CT scans in 11 patients by bedside TCS. Niesen et al.
10) reported that 3 of 25 (12%) cases of SDH were missed when TCS was used, because of insufficient temporal bone windows. In our experience, acoustic artifacts around the skull can be problematic. TCS also has a more limited field of view than CT, which means the operator has to tilt and pan the probe to acquire a complete image of the brain.
710) In Case 2, contralateral EDH on the frontal lobe could have been missed if we had not expected the existence of hematoma due to the presence of a preexisting left frontal bone fracture. Furthermore, because TCS is a user-dependent technique, operators require adequate training regarding accurate evaluations of the brain.
For these reasons, TCS can never replace CT. Nevertheless, we believe intraoperative TCS is a useful point-of-care diagnostic tool, particularly in cases of TBI when time-consuming evaluations are limited because of possible systemic compromise and the need for prompt decision making. A prospective study is needed to improve understanding of the utility and limitations of TCS as compared with CT.
Go to :





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download