Abstract
Objective
Decompressive craniectomy (DC) is a widely used surgical procedure for control of severely increased intracranial pressure in various conditions. The goal of this study is to evaluate the effectiveness of the addition of resection of temporalis muscle and fascia in DC particularly in the treatment of traumatic brain injury.
Methods
Twenty patients underwent temporalis muscle and fascia resection in addition to conventional DC and duroplasty due to massive brain swelling in a single tertiary hospital from 2013 to 2015 were enrolled. Twenty other patients who received the standard techniques by other neurosurgeons in the same period were gathered for the control group. Postoperative computed tomography (CT) as well as functional outcome in both groups were analyzed retrospectively.
Results
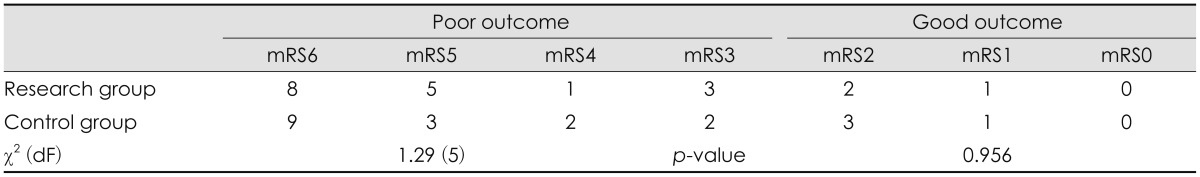
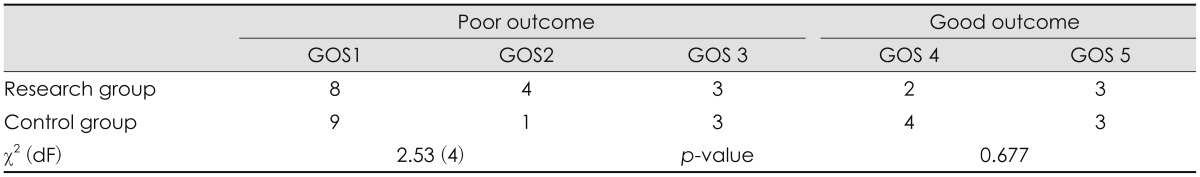
CT volumetry showed a significant increase of 85.19 mL (p<0.001) of extracranial herniation volume in the research group compared with the control group. Using modified Rankin Scale and Glasgow Outcome Scale, there was no statistically significant difference in functional outcome between the two groups.
Go to : 
Decompressive craniectomy (DC) is a surgical procedure involving removal of part of the skull to accommodate a swollen brain without squeezing. It is performed worldwide in patients with severely increased intracranial pressure (ICP) due to several conditions, including traumatic brain injury (TBI), subarachnoid hemorrhage, spontaneous intracerebral hemorrhage (ICH) and malignant hemispheric infarction.914)
Currently, Brain Trauma Foundation2) and the European Brain Injury consortium11) guidelines suggest DC as second-tier therapy for refractory intracranial hypertension that does not respond to conventional therapeutic measures. The standard procedure is the removal of a wide fromtotemporoparietal bone flap measuring at least 12 to 15 cm in diameter, commonly together with expansive duroplasty to maximize brain expansion.717)
However, several modification have been made or are under progression for maximal decompression. One of them is addition of resection of temporalis muscle and fascia in DC. Park et al.15), Park and Hwang14) described the procedure for patients with malignant brain swelling after hemispheric cerebral infarction. It has been used in several institutions including authors', but still lacks clinical evidence for other indications. The goal of this study is to evaluate the efficacy and complications of the technique, in the treatment of TBI.
Go to : 
Among patients with TBI, DC were performed when they developed signs of brain stem compression as a result of increased ICP even though medical treatments for reduction of ICP have been applied, or when massive intra-operative brain swelling is encountered during evacuation of an acute epidural or subdural hematoma. In authors' institution, two neurosurgeons remove temporalis muscle and fascia in addition to standard technique for patients who show severe brain swelling on preoperative computed tomography (CT) or on intraoperative findings.
The inclusion criteria for the current study were 1) TBI, 2) unilateral DC, 3) available pre- and post-operative CT scan, 4) Marshall's grade IV preoperative CT. Patients underwent internal decompression such as lobectomy or removal of traumatic ICH were excluded. Twenty consecutive patients who underwent temporal muscle and fascia resection by two neurosurgeons from 2013 to 2015 were enrolled for the research group. Twenty other patients who received the standard techniques by other neurosurgeons in the same period were gathered for the control group. Patient demographics, clinical histories, initial neurologic status, pre- and post-operative CT scans, and functional outcome of each groups were analyzed and compared retrospectively based on reviews of medical recording and CT.
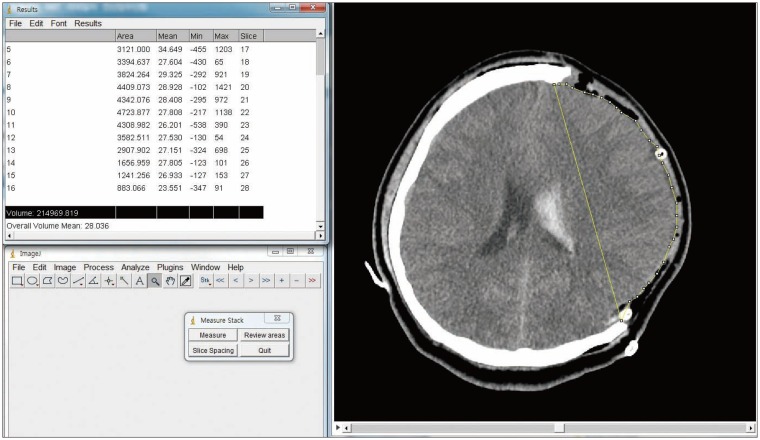
Postoperative CT scans were taken within 24 hours after surgery. The volume of maximal extracranial herniation was measured using CT volumetry. Volumetric analysis was calculated using ImageJ (Version 1.48; National Institutes of Health, Washington, DC, USA). Herniated brain parenchyma on axial brain scans were manually outlined at the virtual line between bony margins of DC and each respective region of interest was calculated, multiplied by slice thickness, and added up using Measure Stack plugin of ImageJ (Figure 1). After discharge, patients were seen in the outpatient clinic. Glasgow Outcome Scale (GOS), modified Rankin Scale (mRS), and presence of postoperative complication was assessed within 1 to 3 months after the operation.
Statistical analyses were performed using SPSS statistics (version 9.3; SPSS Inc., Chicago, IL, USA) All p-values were generated using independent t and chi-square test and p-values less than 0.05 were considered significant.
In accordance with standard DC procedure, a large reverse question mark incision was made. The skin and pericranial flap along with temporal muscle and its fascia, was dissected and elevated as far as the root of the zygoma. After 3 to 4 suitable burr holes were made, craniectomy was performed on frontotemporoparietal bone with a maximum diameter longer than 12 cm. Bone flap margins were follows: anteriorly, anterior to the midpupillary line while avoiding the frontal sinus; posteriorly, 5 cm posterior to an external auditory canal; medially, 2 cm lateral to the superior sagittal sinus; inferiorly, down to the middle cranial fossa. Extensive drilling and rongeuring were performed to the squamous temporal bone for sufficient decompression to prevent uncal herniation.
Dura matter was opened in satellite manner, and subdural hematoma was removed. Careful hemostasis and inspection along with repeated saline irrigation were done for any active bleeding focus and residual hematoma, however, the removal of traumatic ICH or lobectomy were not performed at all. Expansive duroplasty was then performed using artificial dura matter. Hemostasis and multiple marginal tack-up suture were performed to prevent postoperative hematoma.
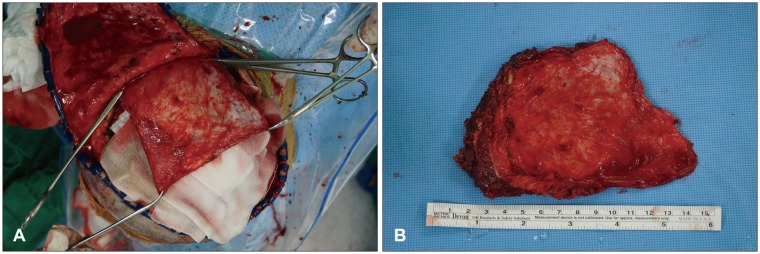
Before closure of incised wounds, temporal muscle and its fascia were bluntly dissected from the skin flap using Metzenbaum scissors or Bovie electrocautery (Figure 2A), and then resected at the level of the floor of middle cranial fossa (Figure 2B). Special attention was paid not to violate course of frontal branch of facial nerve near the zygomatic arch. Supplying arteries including deep temporal arteries were coagulated with bipolar cautery. Following placement of drainage catheters, the scalp was closed layer by layer. Bone flap was sent to the bone bank, and stored at -70℃ for cranioplasty.
Go to : 
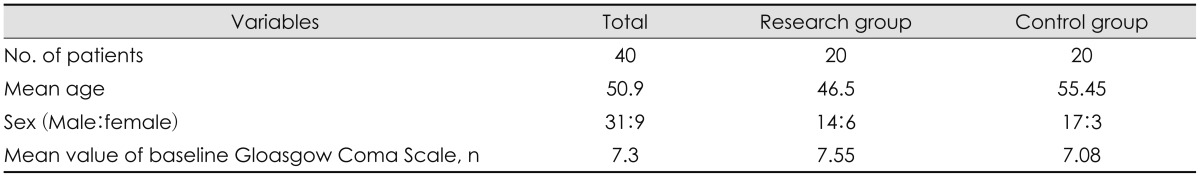
Forty patients (31 men and 9 women, mean age of 50.9 years) were enrolled in the study. Patient demographics are described in Table 1. There was no statistically significant difference in preoperative Glasgow Coma Scale between two groups (p=0.699).
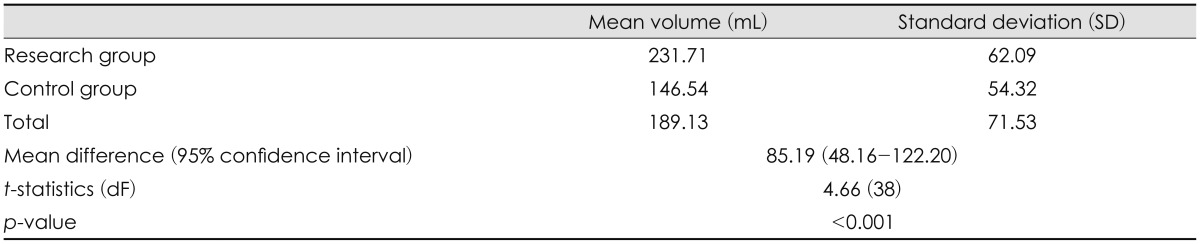
Statistical analysis using independent t-test showed statistically a significant increase (p<0.001) in extracranial herniation volume in the research group. The mean difference between the two groups was 85.19 mL and the rest of the statistic are summarized in Table 2.
The assessment of functional outcome using mRS and GOS in both groups is shown in Table 3 and 4. mRS ranging from 3 to 6 and GOS 1 to 3 were categorized as poor outcome, whereas mRS ranging 0 to 2 and GOS 4 to 5 were categorized as good outcome. Using the chi-square test, there were no statistical difference in outcome between the research and the control group.
Thirty percent of the research group and 25% of the control group reported pain in the operation site more than once in the out-patient follow-up periods, but none of them reported prolonged pain after the cranioplasty. None of the patient in each group reported subjective dysfunction in mastication in ipsi-lateral side.
Only one patient in the research group (0.5%) reported cosmetic discontent after cranioplasty. We performed manually manipulated titanium mesh plate augmentation in cooperation with plastic surgeons, which result in satisfactory cosmetic result for both the patient and physician.
Go to : 
Although its efficacy in improving outcome is still in controversy, DC is widely used as a final effort in patients with increased ICP refractory to medical treatment TBI.7) DC in post-traumatic brain swelling was first introduced by Kocher over 100 years ago17) and many technical modifications have been introduced to maximize its efficacy in reducing ICP. These include subtemporal decompression, circular decompression, fronto- or temporoparietal DC, large frontotemporoparietal DC, hemispheric craniectomy, and bifrontal craniectomy.7) Expansive duroplasty in addition to DC is yet another effort to further reduce ICP and also used and recommended worldwide.7101718) Martin et al.12) even suggested addition of zygomatic arch resection in a recent paper.
The temporal muscle also known as temporalis muscle is a broad, fan-shaped muscle on each side of the head that fills the temporal fossa, superior to the zygomatic arch, inserting into the coronoid process of the mandible.4) This structure, which is familiar to neurosurgeons, is often manipulated during common frontotemporoparietal approaches. Significant effort is made not to damage this muscle to avoid temporomandibular disorder, which could results in pain, masticatory dysfunction, and cosmetic deformity.1319) However, bleeding, contused, swollen muscle, and associated hematoma can cause suboptimal decompression,18) especially in patients with craniofacial injury and temporal base contusion.
In the current study, we explored the possibility of resection of temporal muscle and fascia as a relatively easy, time- and cost-efficient additive technical modification in DC aiming for maximal reduction of increased ICP. In our study, volumetric analysis showed a statistically significant increase in extracranial herniation, reflecting a decrease in ICP.
No statistically significant difference in functional outcome was observed between the two groups in this study, possibly due to small sample size and thus leading to categorization into only two outcome groups in statistical analysis using chi-square test. Further randomized prospective studies with larger sample size are required to assess prognostic benefit with this operative technique.
A major concerns in this procedure is its sequalae, functional and cosmetic abnormalities. Temporal muscle is the most powerful muscle of the temporomandibular joints, functioning in elevation of the mandible. Park et al.15), who examined a similar technique in patients with hemispheric infarction reported no difference in pain and mouth opening. Some decrease in maximal bite force was observed on the affected side, however the chewing function itself was well preserved. The grinding phase of the closure stroke only require one-third of the maximal bite force and the masseter and medial pterygoid muscle could sufficiently compensate.6)
Cosmetic deformity, after temporalis muscle resection could be another major concern in this surgical technique. However, various implants including high density polyethylene implants, polymer bone cement, titanium mesh, or even three-dimensional reconstructed materials are widely available and have shown feasible cosmetic results.351416) In our facility, cranioplasty was routinely performed 1 to 3 months after craniectomy with autogenous bone graft and manually manipulated titanium mesh plate augmentation. Most patients showed minimal cosmetic impairment.
Limitation of current study were that this was relatively small, retrospective study based on chart reviews and subjective surveys, inherently biased. Even though the study revealed increasing volume of extracranial herniation with the technique, but lacks direct measurement of ICP. Further investigations with large, randomized study design with pre- and post-operative measurement of ICP, cerebral blood flow, objective measurements of masticatory function and analysis of various reconstructive methods are required for validation of efficacy and safety of this technique.
Go to : 
In our study, we suggest addition of the resection of temporalis muscle and fascia in DC especially when maximum decompression required for TBI patients. Although there was no difference in functional outcome in both groups, the procedure appears to show a meaningful increase in extracranial herniation volume with minimal masticatory and cosmetic impairment.
Go to : 
References
1. André C, Py Mde O, Niemeyer-Filho P. Temporal muscle haematoma as a cause of suboptimal haemicraniectomy: case report. Arq Neuropsiquiatr. 2003; 61:682–686. PMID: 14513181.

2. Brain Trauma Foundation. Management and prognosis of severe traumatic brain injury. J Neurotrauma. 2000; 24:S1–S106.
3. Choudhry OJ, Christiano LD, Arnaout O, Adel JG, Liu JK. Reconstruction of pterional defects after frontotemporal and orbitozygomatic craniotomy using Medpor Titan implant: cosmetic results in 98 patients. Clin Neurol Neurosurg. 2013; 115:1716–1720. PMID: 23619535.

4. Fehrenbach MJ, Herring SW. Illustrated anatomy of the head and neck. ed 4th. Philadelphia, PA: Saunders;2011.
5. Feng J, Yang C, Cui W. Effectiveness of digital three-dimensional titanium mesh in repairing skull defect under temporalis and reconstructing temporal muscle attachment points. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2014; 28:597–600. PMID: 25073280.
6. Gibbs CH, Mahan PE, Lundeen HC, Brehnan K, Walsh EK, Sinkewiz SL, et al. Occlusal forces during chewing--influences of biting strength and food consistency. J Prosthet Dent. 1981; 46:561–567. PMID: 6946223.

7. Huang X, Wen L. Technical considerations in decompressive craniectomy in the treatment of traumatic brain injury. Int J Med Sci. 2010; 7:385–390. PMID: 21103073.

8. Joo SP, Kim TS, Moon HS. How to minimize ischemic complication related to swollen temporalis muscle following indirect revascularization surgery in moyamoya disease: a technical report. J Neurol Surg A Cent Eur Neurosurg. 2014; 75:231–235. PMID: 23532610.

9. Kakar V, Nagaria J, John Kirkpatrick P. The current status of decompressive craniectomy. Br J Neurosurg. 2009; 23:147–157. PMID: 19306169.

10. Kim DH, Ko SB, Cha JK, Hong KS, Yu KH, Heo JH, et al. Updated Korean clinical practice guidelines on decompressive surgery for malignant middle cerebral artery territory infarction. J Stroke. 2015; 17:369–376. PMID: 26438005.

11. Maas AI, Dearden M, Teasdale GM, Braakman R, Cohadon F, Iannotti F, et al. European Brain Injury Consortium. EBIC-guidelines for management of severe head injury in adults. Acta Neurochir (Wien). 1997; 139:286–294. PMID: 9202767.

12. Martin AG, Abdullah JY, Jaafar A, Ghani AR, Rajion ZA, Abdullah JM. Addition of zygomatic arch resection in decompressive craniectomy. J Clin Neurosci. 2015; 22:735–739. PMID: 25564264.

13. Missori P, Paolini S, Ciappetta P, Seferi A, Domenicucci M. Preservation of the temporal muscle during the frontotemporoparietal approach for decompressive craniectomy: technical note. Acta Neurochir (Wien). 2013; 155:1335–1339. PMID: 23605253.

14. Park J, Hwang JH. Where are we now with decompressive hemicraniectomy for malignant middle cerebral artery infarction? J Cerebrovasc Endovasc Neurosurg. 2013; 15:61–66. PMID: 23844349.

15. Park J, Kim E, Kim GJ, Hur YK, Guthikonda M. External decompressive craniectomy including resection of temporal muscle and fascia in malignant hemispheric infarction. J Neurosurg. 2009; 110:101–105. PMID: 18834267.

16. Park TY, Kim JH, Chang WS, Chang JW, Park YG, Jung HH. Temporal hollowing augmentation with titanium mesh after autologous cranioplasty in temporal muscle resected case: a case report. Korean J Neurotrauma. 2013; 9:154–156.

17. Piek J. Decompressive surgery in the treatment of traumatic brain injury. Curr Opin Crit Care. 2002; 8:134–138. PMID: 12386514.

18. Winn HR. Youmans neurological surgery. ed 5th. Philadelphia, PA: Saunders;2004.
19. Yasuda CL, Costa AL, Franca M Jr, Pereira FR, Tedeschi H, de Oliveira E, et al. Postcraniotomy temporalis muscle atrophy: a clinical, magnetic resonance imaging volumetry and electromyographic investigation. J Orofac Pain. 2010; 24:391–397. PMID: 21197511.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download