Abstract
Objective
Decompressive craniectomy (DC) is a useful surgical method to achieve adequate decompression in hypertensive intracranial patients. This study suggested a new skin incision for DC, and analyzed its efficacy and safety.
Methods
In the retrograde reviews, 15 patients underwent a newly suggested surgical approach using n-shape skin incision technique (Group A) and 23 patients were treated with conventional question mark skin incision technique (Group B). Two groups were compared in the terms of the decompressed area of the craniectomy, protruded brain volume out of the skull layer, the operation time from skin incision to bone flap removal, and modified Rankin Scale (mRS) which was evaluated for 3 months after surgery.
Results
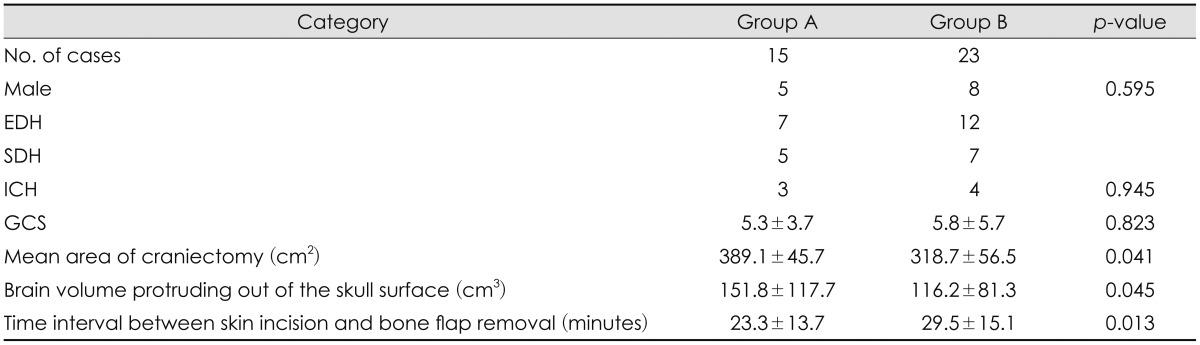
The decompressed area of craniectomy (389.1 cm2 vs. 318.7 cm2, p=0.041) and the protruded brain volume (151.8 cm3 vs. 116.2 cm3, p=0.045) were significantly larger in Group A compared to the area and the volume in Group B. The time interval between skin incision and bone flap removal was much shorter in Group A (23.3 minutes vs. 29.5 minutes, p=0.013). But, the clinical results were similar between 2 groups. Group A showed more favorable outcome proportion (mRS 0-3, 6/15 patients vs. 5/23 patients, p=0.225) and lesser mortality cases proportion 1/15 patients vs. 4/23 patients, but these differences were not significantly observed (p=0.225 and 0.339).
Management of intracranial pressure (ICP) is the main goal for patients with traumatic brain injury. Moreover, direct injury, secondary insult due to post-traumatic increase of ICP or decrease in cerebral perfusion pressure are well recognized as potential causes of increasing mortality and morbidity.467) Decompressive craniectomy (DC) is an important method to decreased ICP,9) and DC is widely performed throughout the world.10) This surgical procedure is performed by any neurosurgeons or even residents under training, so it should achieve enough decompression without interoperator differences, but textbook only commented that "the bone flap should not be just as large as possible".3) The authors considered that the skin incision could interfere the size of enough decompression area, and considered new skin incision technique what we called the "n-shaped skin incision". The purpose of this study is to suggest a new skin incision technique for decompressive surgery to promote greater decompression, and compared the efficacy and safety of this new technique compared to the conventional surgical technique.
This retrograde review study was conducted among patients suffering from severe traumatic brain injury. The surgical indication of space-occupying severe traumatic brain injury was as follows: a midline shift of the cerebral structures and parenchymal hypodensity in greater than 50% of the middle cerebral artery territory, which are both identified on computed tomography (CT) scanning.25) A total of 38 patients, who underwent decompressive surgery in single university hospital between May 2012 and December 2014, were recruited for this study. These patients were classified into 2 groups: Group A (n=15) which the patients were underwent n-shaped skin incision, and Group B (n=23) which the patients who underwent the conventional surgical method (question mark skin incision). All patients were managed promptly after the surgical intervention, such as the medical treatment and acute care from admission to post-operation.
The overall surgical method was similar except the skin incision techniques. All patients were endotracheal intubated under general anesthesia. In Group A, an "n-shaped" skin incision was made and included the slightly curved line along the frontal hair line and the parabola line pass through the parietal and temporal regions on the operation side as presented in Figure 1A. In Group B, a large skin incision was made and included the frontal, parietal, and temporal regions on the side which similar shape to question mark, and the incision was made as wide as possible (Figure 1B). The soft tissue flap was elevated and retracted by the standard techniques in both groups, and the dura was fixed at the edge of the craniotomy to prevent epidural bleeding. The dura was opened with a large asterisk-shaped incision that involved the frontal, parietal, and temporal lobes. Lyophilized cadaver dura was placed underneath the incised dura, and it was secured with several sutures. The bone flap was frozen and stored.
The area of the decompressed regions (cm2) (Figure 2A), protruded brain volume (cm3) (Figure 2B) out of the skull surface, and the time interval between skin incision and bone flap removal were compared. The area of the decompression region was approximately calculated by the following equation: A (area, cm2)=D * d * π; "D" was the anteroposterior diameter of the bone flap; "d" was the perpendicular diameter to D from the superior craniectomy margin to the inferior margin in centimeters. The protruded brain volume protruding out of the skull surface is simply calculated by the following equation: V (volume, cm3)= 1/3 * A * H; "A" is the area of the bone flap; and "H" is the height of the outward of the brain on CT from the level of the center of the bone flap in centimeters. The time interval between skin incision and decompression time was calculated using the anesthetic medical record (minutes).
Patients' outcomes were clinically evaluated according to the modified Rankin Scale (mRS) for 3 months after surgery. Surgical mortality was defined as death within 30 days after the procedure. A favorable outcome and poor outcome were defined as mRS 0 to 3 (moderate disability or better), and mRS 4 to 6 (severe disability or death). All values were processed with SPSS 12.0 software (SPSS Inc., Chicago, IL, USA) and are expressed as the mean±standard deviation (SD). The measurements and outcomes between 2 groups were analyzed by Mann-Whitney U test and chi-square test. Statistical significance was set at p lesser than 0.05.
Table 1 summarized the demographic data for both groups. No significant differences between the 2 groups regarding age, sex, diagnosis, and the initial neurologic scores were observed. The area of craniectomy was larger in Group A (389.1±45.7 cm2) than that in Group B (318.7±56.5 cm2, p=0.041). The protruded brain volume out of the skull surface was 151.8±117.7 cm3 in Group A and 116.2±81.3 cm3 in Group B, indicating that more decompression was performed in Group A (p=0.045). In addition, the time interval between skin incision and bone flap removal was much shorter in Group A (23.3±13.7 minutes vs. 29.5±15.1 minutes,
p=0.013).
There were no surgery-related complications except for 1 patient in Group A and 3 patients who had a postoperative epidural hematoma (EDH) and subsequent EDH evacuation (p=0.531). The clinical outcomes throughout the 3 month after surgery were as follows: In Group A, 6 (40.0%) out of 15 patients had favorable outcomes (mRS 0-3) and 1 (6.7%) patient had expired; in Group B, 5 (21.7%) out of 23 patients had favorable outcomes and 4 (17.4%) patients had expired. It was considered that Group A showed more favorable outcome proportion and lesser mortality cases proportion, but these differences were not significantly observed (p=0.225 and 0.339) due to the limitation of the number of cases.
The role of surgery in the management of traumatic brain injury was often based on subjective criteria or previous experience of each individual surgeon.11) Surgical evacuation
should be considered when the neurological status which revealed the development of new "brainstem" signs and a sustained increase in ICP were suspected. Image studies should be performed urgently to evaluate the progression of intracerebral hemorrhage (ICH) and brain swelling. An increase in the size of ICH on image studies is also an indication for surgical removal. Decompression could provide an enlargement of the intracranial space, preventing further rise of ICP and cerebral herniation.8) A craniectomy of greater than 8 cm may be large enough, but to obtain a real decompression, most authors recommend a minimum diameter of 12 cm.
Intraoperative surgical technique is important in achieving adequate decompression for craniectomy. However, skin incision may also play a critical role in the whole hemicranium.1) The most well-known common technical skin incision technique is the classical "question mark" flap (Figure 1B). The incision starts at the temporal level, which then goes toward the superior level to preserve blood supply of the flap from the superficial temporal artery. The temporal muscle is dissected in one plane and attached to the flap. The advantage of this incision is that most neurosurgeons are accustomed to the anatomical structures; however, in the posterior segment of the flap, the blood supply is low, which may lead to several complications, especially dehiscent wound or skin necrosis. Indeed, the desired decompression size is achieved by various extensions of the scalp to the outer edge of the incision. However, our newly supposed "n-shape" incision method shows that the desired decompression has easily been achieved with less time until bony decompression (Figure 2). This n-shape skin incision included the slightly curved line along the frontal hair line and the parabola line pass through the parietal and temporal regions on the operation side as presented in Figure 1A. In this study, we indicated that the "n-shape" skin incision method has several advantages, such as larger mean area of craniectomy, more brain volume protruding out of the skull surface, and faster time interval between skin incision and bone flap removal. This incision technique could also provide minimized ischemic complication at the operatory wound site due to the preservation of the occipital artery, and this may be a better blood supply, as presented in Figure 3.1)
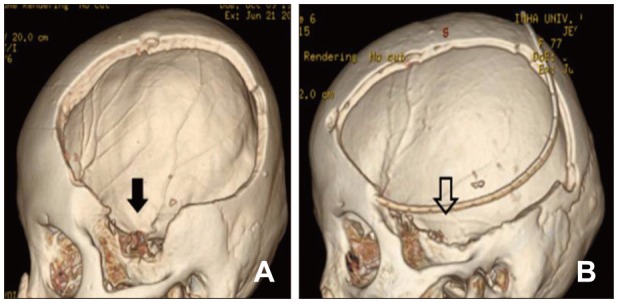
There was also greater improvement in the clinical outcomes using this new skin incision method. The clinical outcomes at 3 months' post-surgery was as follows: in Group A, 6 (40.0%) out of 15 patients had favorable outcomes (mRS 0-3) and 1 (6.7%) patient had expired, whereas in Group B, 5 (21.7%) out of 23 patients had favorable outcomes and 4 (17.4%) patients had expired. We consider this to be an excellent clinical result with respect to two decompressive factors, such as a wider decompression size and greater possibility of decompression such as temporal bone. As well known to neurosurgeons, the vital brain structure is located at the area of the temporal bone; hence, decompression of this site is crucial to prevent central herniation. The conventional skin incision could provide a visualization of this area; however, the thickness of the temporalis muscles makes it very difficult. Our new incision method, nonetheless, included this critical point in the incision line, so the vital temporal bone decompression was easier and faster to the conventional method (Figure 4). Indeed, surgery-related complications with the new incision technique showed similar results compared with the conventional technique: 1 (6.6%) patient in Group A vs 3 patients (13.0%) in Group B with postoperative EDH and subsequent EDH evacuation. The crossed line point could be a problem in scalp wound healing, but in this retrospective study, there were only minimal skin problems which were improved after simple dressing.
Although this study showed excellent results from using the new skin incision method for DC, there are still limitations worth noting for this study. First, this study included only a small number of cases and was retrograde reviewed. So many possible contributing factors were ignored in this study. Secondly, the calculated decompressive size was measured using CT images; hence, a delicate volume to achieve desired decompression was not exactly presented. Despite this limitation, this study provided notable surgical results and clinical outcomes of the "n-shape" skin incision method for DC. Future studies are necessary to further evaluate this new technique; we, however, believe that this surgical technique would show excellent results, similar to our study, for well-designed studies in the future.
References
1. Balan C, Alliez B. Decompressive craniectomy: from option to standard - Part I. Rom Neurosurg. 2009; 16:20–26.
2. Cho DY, Chen TC, Lee HC. Ultra-early decompressive craniectomy for malignant middle cerebral artery infarction. Surg Neurol. 2003; 60:227–232. PMID: 12922040.

3. Chung J, Bang OY, Lim YC, Park SK, Shin YS. Newly suggested surgical method of decompressive craniectomy for patients with middle cerebral artery infarction. Neurologist. 2011; 17:11–15. PMID: 21192185.

4. Juul N, Morris GF, Marshall SB, Marshall LF. Intracranial hypertension and cerebral perfusion pressure: influence on neurological deterioration and outcome in severe head injury. The Executive Committee of the International Selfotel Trial. J Neurosurg. 2000; 92:1–6.
5. Lanzino DJ, Lanzino G. Decompressive craniectomy for space-occupying supratentorial infarction: rationale, indications, and outcome. Neurosurg Focus. 2000; 8:e3.

6. Miller JD, Becker DP, Ward JD, Sullivan HG, Adams WE, Rosner MJ. Significance of intracranial hypertension in severe head injury. J Neurosurg. 1977; 47:503–516. PMID: 903804.

7. Oh CH, Shim YS, Yoon SH, Hyun D, Park H, Kim E. A Single Center Retrospective Review of 10 Years. Korean J Neurotrauma. 2016; 12:11–17. PMID: 27182496.
8. Rahmanian A, Seifzadeh B, Razmkon A, Petramfar P, Kivelev J, Alibai EA, et al. Outcome of decompressive craniectomy in comparison to nonsurgical treatment in patients with malignant MCA infarction. Springerplus. 2014; 3:115. PMID: 24711983.

9. Timofeev I, Czosnyka M, Nortje J, Smielewski P, Kirkpatrick P, Gupta A, et al. Effect of decompressive craniectomy on intracranial pressure and cerebrospinal compensation following traumatic brain injury. J Neurosurg. 2008; 108:66–73. PMID: 18173312.

10. Wen L, Wang H, Wang F, Gong JB, Li G, Huang X, et al. A prospective study of early versus late craniectomy after traumatic brain injury. Brain Inj. 2011; 25:1318–1324. PMID: 21902550.

11. Zacko JC, Harris L, Bullock R. Surgical management of traumatic brain injury. In : Winn HR, editor. Youmans neurological surgery. ed 6. Philadelphia, PA: W. B. Saunders;2011. p. 3424–3452.
FIGURE 1
(A, B) Skin incision variation between Group A (n-shaped incision) and Group B (large question mark incision), including the frontal, parietal, and temporal regions.
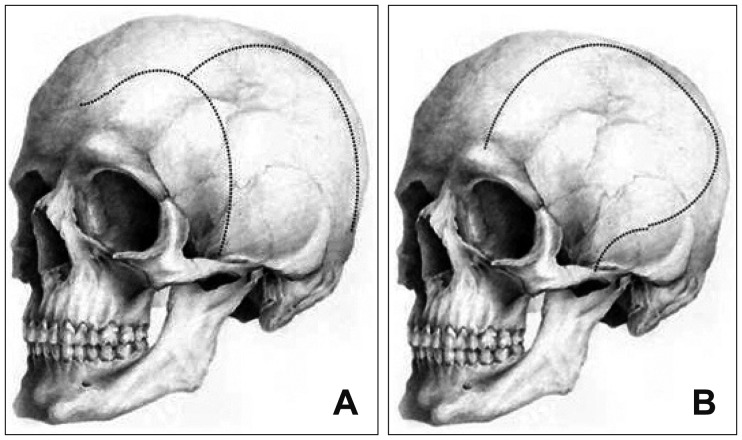
FIGURE 2
The area of the decompression region (A) and the protruded brain volume (V) protruding out of the skull surface were approximately calculated by the following equation: A (area, cm2)=D * d * π; "D" was the anteroposterior diameter of the bone flap; "d" was the perpendicular diameter to D from the superior craniectomy margin to the inferior margin in centimeters; V (volume, cm3)=1/3 * A * H; "A" is the area of the bone flap; and "H" is the height of the outward of the brain on computed tomography from the level of the center of the bone flap in centimeters.
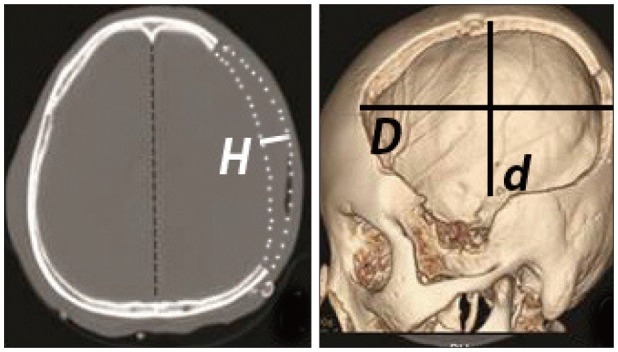
FIGURE 3
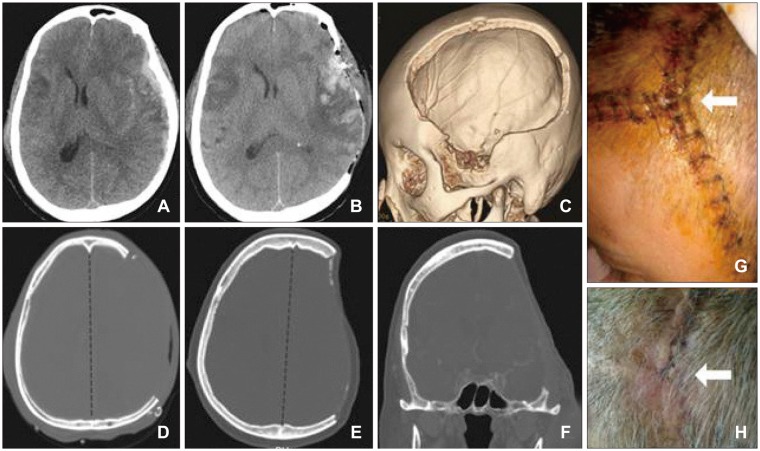
Case illustration of skin incision in Group A with n-shape skin incision, which included the frontal, parietal, and temporal regions: (A) acute subdural hematoma before surgery; (B) immediate postoperative status; (C-F) 3d-reconstructed decompressed bone area; (G, H) possible skin problem area where 2 lines were crossed (arrows), but most case were improved after simple dressing.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download