Introduction
The number of patients taking antiplatelet agents is increasing because antiplatelet therapy has been shown to have a clear advantage in secondary prevention of cardiovascular events and a possible benefit in primary prevention as well.
15) However, in 1994, it was reported that preoperative use of antiplatelet agents was the most commonly associated risk factor for postoperative hematomas requiring surgical evacuation after neurosurgery.
13) Bleeding during or after neurosurgical operations is sometimes disastrous. Therefore, most neurosurgeons recommend that all antiplatelet agents should be discontinued for a few days before surgery.
15)
Decompressive craniectomy (DC) is performed to decrease intracranial pressure (ICP) and to increase cerebral perfusion pressure in patients with intractable ICP resulting from various intracranial lesions.
2414) DC can be a life-saving measure in patients with intractable ICP.
4) In the majority of traumatic brain injury (TBI) cases, DC is performed emergently. Therefore, DC cannot be delayed to the time when the effect of antiplatelet agents on bleeding tendency dissipates. Hemorrhagic complications are one of the major complications of DC.
218) Antiplatelet therapy before the onset of cerebral infarction is a risk factor for postoperative hemorrhagic complications after DC for stroke.
17) However, the effect of preinjury antiplatelet therapy on hemorrhagic complications after DC in patients with TBI is rarely studied.
19) In the present study, we evaluated the effect of antiplatelet therapy on hemorrhagic complications after emergent DC in patients with TBI.
Go to :

Materials and Methods
We retrospectively investigated patients with TBI who underwent emergent DC on arrival at the emergency department between 2006 and 2015. Patients who met all of the following criteria were included in the present study: 1) Glasgow Coma Scale 13 or less, 2) traumatic intracerebral hemorrhage (ICH), hemorrhagic contusion, or subdural hemorrhage (SDH) in brain computed tomography (CT) scan, and 3) a midline shift of at least 5 mm. Patients who did not meet any of the following criteria were excluded: 1) no brain stem reflex, 2) platelet count of less than 100 k/µL, 3) prothrombin time international normalized ratio (PTINR) of more than 1.4, 4) activated partial thromboplastin time (aPTT) of more than 40 seconds, or 5) medically unstable condition.
In the DC procedure, after hair shaving and preparation of the skin, a standard frontotemporoparietal hemicraniectomy with at least diameter of 12 cm bone flap was performed. The dura mater was opened, and evacuation of hematoma was performed when necessary. A dura substitute was then placed over the cerebral cortex and the opened dura was repositioned over the substitute. The bone flap was not replaced. An ICP monitor was routinely installed in the epidural space. A subgaleal drain was also routinely placed. The temporalis muscle, galea, subcutaneous tissues, and the scalp were closed in a layer by layer fashion. DC was performed by 5 different surgeons.
Brain CT scan was performed in the immediate postoperative period and on postoperative 7 days. In case of ICP of more than 25 mmHg, an emergent CT scan was performed at any time. Hemorrhagic complication was defined when at least one of the following criteria was met: 1) Epidural hemorrhage (EDH) or SDH with a thickness of 10 mm or more within the field of DC in the immediate postoperative CT scan, 2) increase in the thickness of EDH or SDH within the field of DC in the CT scan within or on postoperative 7 days compared with the thickness of EDH or SDH in the immediate postoperative CT scan (
Figure 1), and 3) progression of preexisting contusion or ICH relating to the edge of the DC or within the field of DC in the CT scans, or 4) new areas of hemorrhagic contusion or ICH relating to the edge of the DC or within the field of DC in the CT scans (
Figure 2).
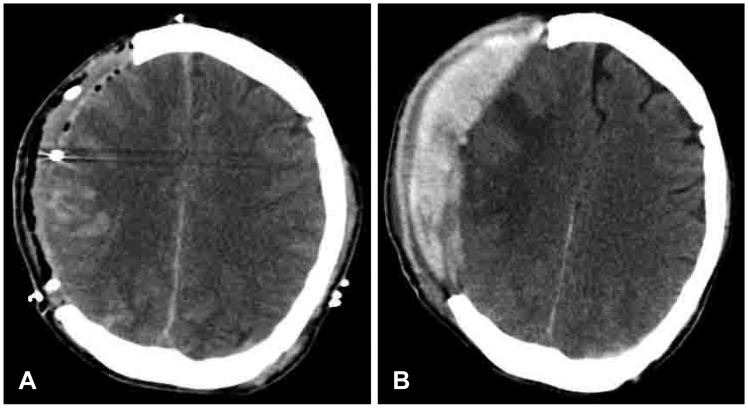 | FIGURE 1(A) The immediate postoperative computed tomography (CT) scan shows epidural hemorrhage (EDH) of maximal thickness of less than 10 mm. (B) CT scan performed when the intracranial pressure was more than 25 mmHg shows increased thickness of EDH compared with that in the immediate postoperative CT scan.
|
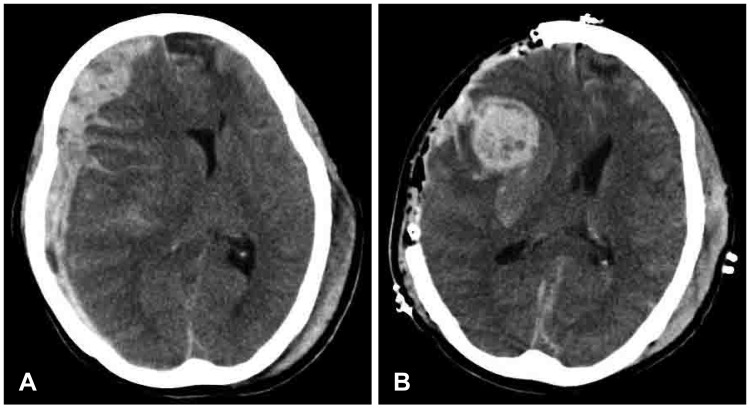 | FIGURE 2(A) Preoperative computed tomographic (CT) scan shows no hemorrhagic contusion. (B) The immediate postoperative CT scan shows progression of hemorrhagic contusion within the field of decompressive craniectomy.
|
Reoperation was performed in any of following cases: 1) EDH or SDH with a thickness of 10 mm or more within the field of DC in the immediate postoperative CT scan or 2) when ICP was more than 25 mmHg and hemorrhagic complication was identified in the CT scan.
Patients were separated into two groups based on antiplatelet therapy before TBI: group 1 (patients taking antiplatelet agent) and group 2 (patients not taking antiplatelet agent). The demographic data, clinical parameters including injury severity score (ISS), and laboratory findings, including platelet (PLT) count, PTINR, aPTT, and fibrinogen, were obtained retrospectively from the patients' electronic medical records and were compared between the two groups.
5) The rate of hemorrhagic complications and reoperation due to hemorrhagic complications within 7 days after DC were compared between the two groups.
Statistical analysis
To compare categorical variables, a chi-square test or Fisher exact test were used. We used the Student's t-test for continuous variables. SPSS Statistics (version 20; IBM Corp., Armonk, NY, USA) was used for statistical analysis and a p-value of 0.05 or less was considered statistically significant.
Go to :

Results
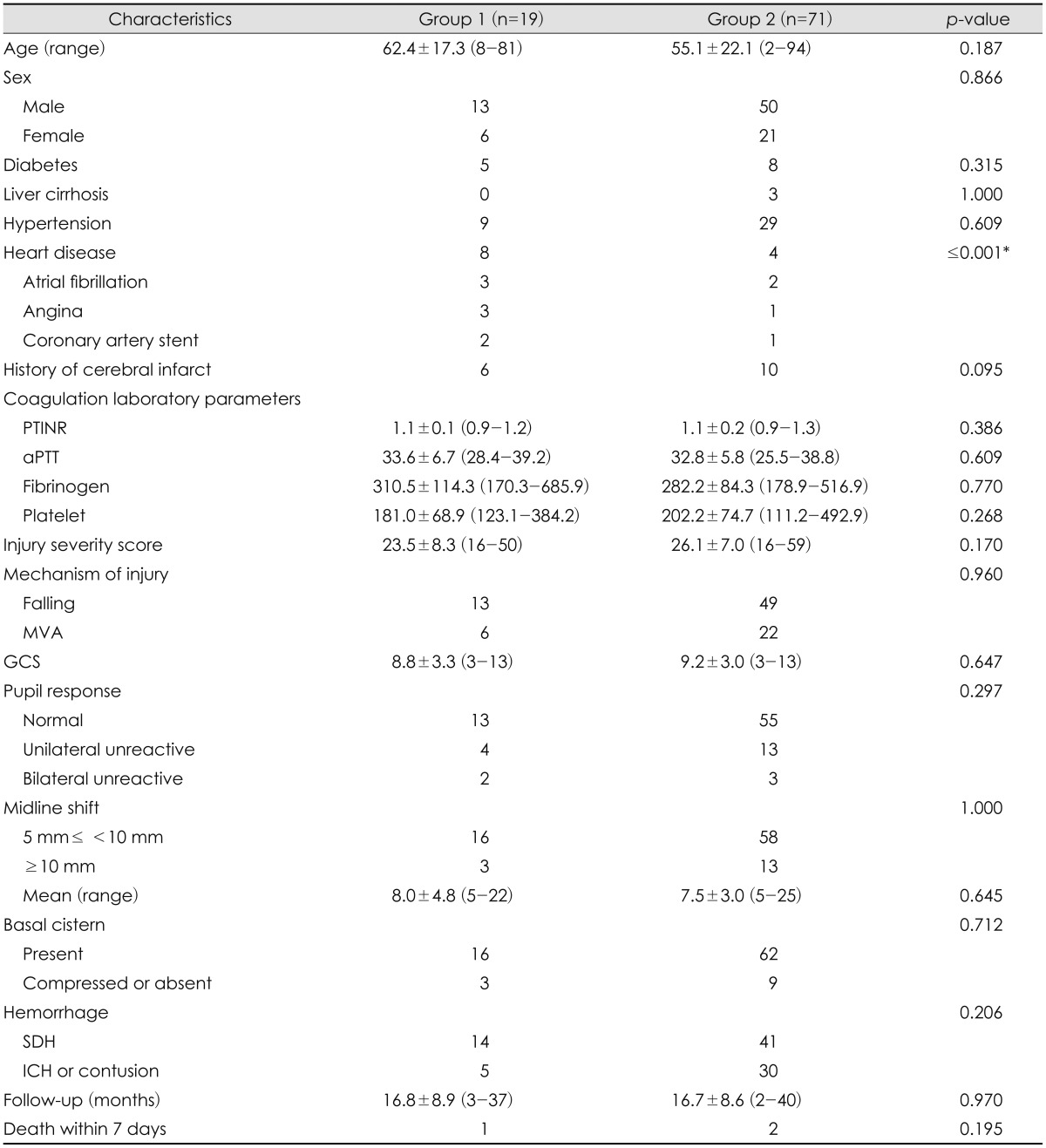
During the study period, emergent DC on arrival at the emergency department was performed in 90 patients with TBI. Of them, 19 patients were taking antiplatelet agent before TBI. Twelve patients were taking acetylsalicylic acid (ASA), 4 clopidogrel, and 3 dual antiplatelet agents of ASA and clopidogrel. The purpose of antiplatelet therapy was prevention of cerebrovascular disease in 6 patients, prevention of ischemic heart disease in 8, and secondary prevention of atrial fibrillation in 3. Two patients took antiplatelet agent for secondary prevention of ischemic heart disease after coronary artery stent. The history of heart diseases such as atrial fibrillation, angina, or history of coronary artery stent was different between the two groups. Demographic parameters, PLT count, PTINR, aPTT, fibrinogen, ISS, and medical diseases or medications that could affect bleeding tendency, such as liver cirrhosis, were not different between the two groups (
Table 1).
TABLE 1
Characteristics of the patients

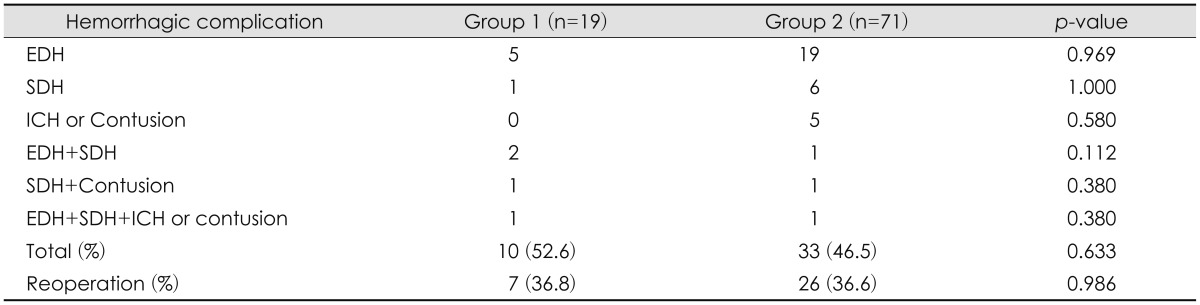
The rate of hemorrhagic complications was 52.6% (10/19) in group 1 and 46.5% (33/71) in group 2. The most common hemorrhagic complication was postoperative EDH in 24 patients followed by SDH in 7 and hemorrhagic contusion or ICH in 5 (
Table 2). Two or more hemorrhagic complications occurred in 7 patients (
Table 2). Although the rate of hemorrhagic complications within 7 days after emergent DC was higher in group 1, no statistical difference was found between the two groups (
p=0.633) (
Table 2). The rate of hemorrhagic complications was not associated with the type of traumatic hemorrhage before DC in group 1. The rate of hemorrhagic complications that required reoperation was 36.8% (7/19) in group 1 and 36.6% (26/71) in group 2. Reoperations were performed on average 2.0 days (range, 1-5 days) after DC in group 1, and 1.9 days (range, 0-7 days) after DC in group 2. Although the rate of hemorrhagic complications that required reoperation was higher in group 1, no statistical difference was found between the two groups (
p=0.986) (
Table 2). In patients with preinjury antiplatelet therapy, statistically significant risk factors that affect the rate of hemorrhagic complications or the rate of reoperation were not found. One patient in group 1 and 2 in group 2 died from hemorrhagic complications within 7 days after DC. In patients with preinjury antiplatelet therapy, atrial fibrillation occurred in 1 patient 8 days after DC. However, this was not fatal and atrial fibrillation subsided after regular treatment with beta-blocker.
TABLE 2
Postoperative hemorrhagic complication

Go to :

Discussion
In patients with TBI, the effect of antiplatelet agent on the expansion of hemorrhage or the requirements of neurosurgery remains controversial. Cull et al.
6) reported that antiplatelet therapy in patients with TBI aged 40 years and older showed no difference in ICHs that required neurosurgical evacuation and in the in-hospital death rate, as compared to patients not taking antiplatelet agent. Conversely, Joseph et al.
10) reported that patients with traumatic ICH on preinjury clopidogrel had a tendency to have progression of hemorrhage that required neurosurgical evacuation. Batchelor and Grayson
3) reported a slight increase in the mortality in patients with blunt TBI who had taken antiplatelet agent before TBI in their meta-analysis. However, the results did not show statistically significant difference.
3)
With regard to the effect of antiplatelet agents on postoperative hemorrhage, taking ASA before operation and during the early postoperative period had no significant influence on the postoperative mortality and morbidity from myocardial infarction, but increased the rate of major postoperative hemorrhagic complications in patients who had undergone noncardiac surgery in the Perioperative Ischemic Evaluation-2 (POISE-2) trial.
7) In this trial, the rate of major bleeding was higher in the ASA group than in the placebo group (4.6% vs. 3.8%;
p=0.04).
7) However, the results in the neurosurgical operation were unclear, because the authors did not describe "other procedure" in detail. According to the 2014 American College of Cardiology/American Heart Association guideline, initiation or continuation of ASA is not helpful in patients undergoing elective noncardiac noncarotid operation, except in patients who have had previous coronary artery stent, unless the risk of ischemic insults is higher than that of postoperative hemorrhage.
8) A recent study using platelet functional assays demonstrated that the mean time for complete reversal of platelet reactivity is 2.6 days in patients who take low-dose ASA and 2.8 days in those who take clopidogrel.
9) The authors recommended that the appropriate time for noncardiac procedures after stopping antiplatelet agents be less than 5 days.
9)
DC in patients with TBI was performed emergently in most cases, therefore, DC could not be delayed to the time when the effect of antiplatelet agents disappeared. Hemorrhagic complications of neurosurgical procedures are sometimes disastrous, which is why most neurosurgeons recommended that all antiplatelet agents should be discontinued before surgery.
15) In 1994, Palmer et al.
13) reported that the perioperative administration of ASA in intracranial surgery was the most commonly associated risk factor for postoperative hemorrhagic complications requiring additional surgery for hematoma evacuation. They reported the rate of surgery for evacuation of postoperative hemorrhage was 1.1% in neurosurgical patients and 43% of patients requiring surgery for evacuation of postoperative hemorrhage were taking an antiplatelet agent before surgery.
13) In 2007, in a survey of practicing German neurosurgeons, 94 (66.2%) responders considered that patients who were taking low-dose ASA were at increased risk for perioperative hemorrhage, and 73 (51.4%) reported personal experience of perioperative hemorrhage.
11) However, in more recent studies, contrary results were reported and conflicting conclusions were drawn about the management of neurosurgical patients on antiplatelet agents. A recent study in 2013 showed the safety of the perioperative use of ASA in a 67-year-old woman who underwent surgical removal of a meningioma in the middle fossa after embolization of the middle meningeal artery.
16) She had a history of placement of a drug-eluting coronary artery stent 3 months before the surgery and no unusual surgical bleeding occurred during resection of the meningioma.
16) In a recent prospective study of a cohort of 100 neurosurgical patients on antiplatelet therapy who underwent spine surgery, there was no increase in the risk of postoperative spinal hemorrhage with the use of ASA or clopidogrel.
1) The authors suggested that spine surgery could be done without stopping antiplatelet therapy.
1) In 2015, Rahman et al.
15) investigated 83 patients with brain tumor on chronic ASA therapy. Fifty five patients had discontinued ASA before surgery and 28 patients continued ASA during the perioperative period.
15) There were no statistical differences between the groups in outcomes, including bleeding complications, need for reoperation, or thrombotic complications in this retrospective cohort analysis.
15) Ogawa and Tominaga
12) reported 15 consecutive patients with sellar and parasellar tumors who had undergone transsphenoidal surgery without discontinuation of antiplatelet agent or anticoagulant therapy in 2015. In this study, the patients who continued ASA during the perioperative period demonstrated a trend for increased blood loss during the surgery.
12) However, this did not result in increased risk of postoperative hemorrhage or the rate of reoperation.
12) With regard to the effect of antiplatelet agents on postoperative hemorrhage after DC, Schuss et al.
17) reported that preoperative use of ASA was the only risk factor in patients with acute ischemic stroke. However, they did not describe the type of hemorrhagic complications and the rate of reoperation.
17) Conversely, Sturiale et al.
19) reported that antiplatelet agent was not significantly associated with increased postoperative hemorrhagic contusion after DC in patients with TBI.
In the present study, although the rate of hemorrhagic complications and the rate of reoperation due to hemorrhagic complications were higher in patients on preinjury antiplatelet agent, no statistical difference was found compared to the patients who were not on preinjury antiplatelet agent. Therefore, emergent DC in patients with TBI should not be delayed or hesitated because of preinjury antiplatelet therapy.
The present study was a retrospective study in one institute. Thus, the study could have weaknesses, including loss of patient information. However, in patients with TBI, preinjury antiplatelet therapy did not significantly influence hemorrhagic complications after DC. We hope that our research will form the basis for further studies designed to evaluate the effect of preinjury antiplatelet therapy on hemorrhagic complications after DC in patients with TBI.
Go to :






 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download