This article has been
cited by other articles in ScienceCentral.
Abstract
Objective
Symptomatic epidural fluid collection (EFC) arising as a complication of cranioplasty is underestimated and poorly described. The purpose of this study was to investigate the risk factors for development of symptomatic EFC after cranioplasty following traumatic brain injury (TBI).
Methods
From January 2010 to December 2014, 82 cranioplasties following decompressive hemicraniectomy for TBI were performed by a single surgeon. Of these 82 patients, 17 were excluded from this study due to complications including postoperative hematoma, hydrocephalus, or infection. Sixty-five patients were divided into 2 groups based on whether they had developed symptomatic EFC: 13 patients required an evacuation operation due to symptomatic EFC after cranioplasty (Group I), and 52 obtained good outcome without development of symptomatic EFC (Group II). We compared the 2 groups to identify the risk factors for symptomatic EFC according to sex, age, initial diagnosis, timing of cranioplasty, cerebrospinal fluid (CSF) leakage during cranioplasty, size of bone flap, and bone material.
Results
A large bone flap and CSF leakage during cranioplasty were identified as the statistically significant risk factors (p<0.05) for development of symptomatic EFC. In Group I, 11 patients were treated successfully with 5 L catheter drainage, but 2 patients showed recurrent EFC, eventually necessitating bone flap removal.
Conclusion
A larger skull defect and intraoperative CSF leakage are proposed to be the significant risk factors for development of symptomatic EFC. Careful attention to avoid CSF leakage during cranioplasty is needed to minimize the occurrence of EFC, especially in cases featuring a large cranial defect.
Go to :

Keywords: Decompressive craniectomy, Cranioplasty, Fluid, epidural, Complication
Introduction
Decompressive craniectomy (DC) is a lifesaving surgery for malignant brain swelling resulting from severe head injuries.
1) Patients who survive following a DC commonly require cranioplasty to repair skull defects, both for cosmesis and improved neurological function.
Although cranioplasty is regarded as a relatively simple procedure in theory, the procedure can give rise to serious complications, such as infection, postoperative hematoma, bone resorption, recurrent seizures, and subdural or epidural fluid collection (EFC).
34678)
Among these complications, EFC following cranioplasty is not well described, and its significance is yet to be ascertained, with only limited reports available on the same. Thus far, EFC is reported to be a relatively rare complication after cranioplasty, and its natural course is known to be one of spontaneous regression or disappearance.
711)
To the best of the authors' knowledge, no previous published reports have evaluated symptomatic EFC after cranioplasty. This study discusses the possible pathological mechanisms and risk factors underlying symptomatic EFC following cranioplasty.
Go to :

Materials and Methods
We conducted a retrospective study, evaluating a total of 82 patients who underwent cranioplasty following decompressive hemicraniectomy for traumatic brain injury (TBI), performed by a single neurosurgeon at our institute between January 2010 and December 2014.
We excluded 17 patients who had postoperative hematoma, hydrocephalus, or infection eventually necessitating removal of bone flap, and a total of 65 patients were enrolled in this study (
Table 1).
TABLE 1
Demographic data of 82 patients who underwent cranioplasty for traumatic brain injury

These 65 patients were divided into 2 groups based on whether they had developed symptomatic EFC: 13 patients required evacuation drainage by 5 L catheter due to development of symptomatic EFC after cranioplasty (Group I), and 52 patients obtained a good outcome without development of symptomatic EFC (Group II). In cases of asymptomatic small amounts of EFC, patients were classified as Group II. The symptomatic EFC in Group I was evacuated through one burr hole using a 5-L catheter under local an-esthesia.
In all patients, serial brain computed tomography (CT) scans were taken to enable evaluation of EFC after cranioplasty. The cranioplasties were performed as per standard procedure, by a single neurosurgeon at our institute. Most cranioplasties were performed using an autologous bone flap. The bone flap was fixed using microscrews and miniplates. The autologous bone flap was frozen and stored prior to surgery at -78℃. A closed drainage system was maintained in all patients for 2 to 3 days.
The following variables were analyzed to determine their contribution to the risk of development of symptomatic EFC: age, sex, initial diagnosis, time interval between craniectomy and cranioplasty, cerebrospinal fluid (CSF) leakage during cranioplasty, and size of bone flap.
Statistical analysis
Mean values and standard deviation were calculated for all measurements. Associations between categorical variables were explored using Fisher's exact test instead of a chi-square test, due to the study's relatively small sample size. p values less than 0.05 were considered statistically significant.
Go to :

Results
Sixty-five patients (45 male, 20 female) who underwent cranioplasty after DC for TBI were included in this study. Among them, 13 patients needed to undergo drainage procedures due to symptomatic EFC presenting with mental deterioration or motor weakness. These patients were assigned to Group I. Fifty-two patients did not require any drainage procedures, and they were assigned to Group II.
Eleven patients in Group I showed complete resolution of EFC following the drainage procedure (
Figure 1). Two patients with intraoperative CSF leakage showed recurrent EFC in spite of the drainage procedure, eventually necessitating the removal of a bone flap (
Figure 2).
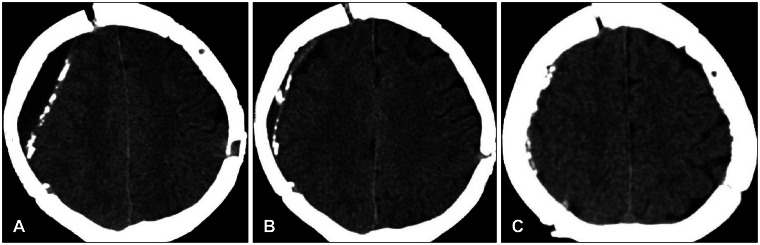 | FIGURE 1A 62-year-old man showing aggravated left side motor weakness for symptomatic epidural fluid collection (EFC). A: Computed tomography (CT) scan 4 days post-cranioplasty show EFC. B: Evacuation of fluid through a 5 L catheter, the collected fluid was completely removed. C: CT scan taken 3 months after cranioplasty reveal no recurrent fluid.
|
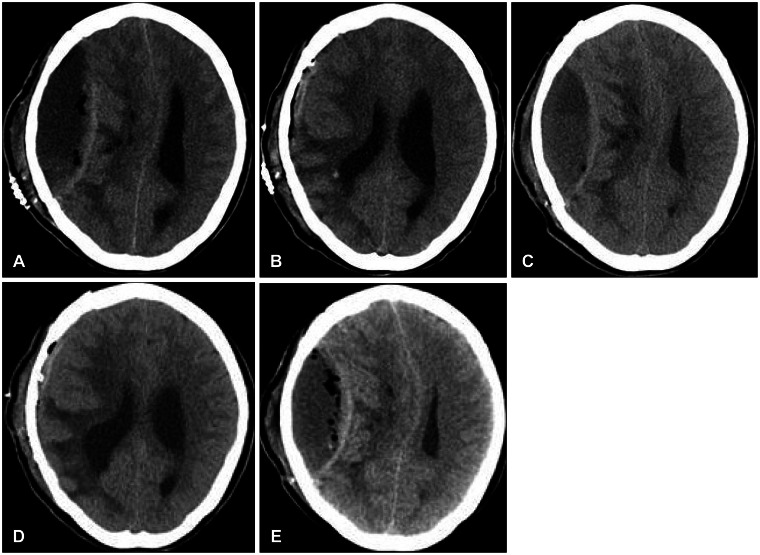 | FIGURE 252-year-old woman eventually requiring removal of the bone flap caused by recurrent epidural fluid collection (EFC). A: Computed tomography (CT) scan 5 days post-cranioplasty; EFC is seen in the epidural space. Midline shift is evident, as is the presence of air bubbles. B: Evacuation of fluid through a 5 L catheter. The mass effect with midline shift is resolved. C: Four days after removal of the catheter, a CT image shows recurrent EFC with significant midline shift. D: The collected fluid was removed again with a 5 L catheter. E: Three days after the second trephination procedure, EFC was observed at the same lesion site; significant midline shift and a large number of air bubbles are evident in the CT image.
|
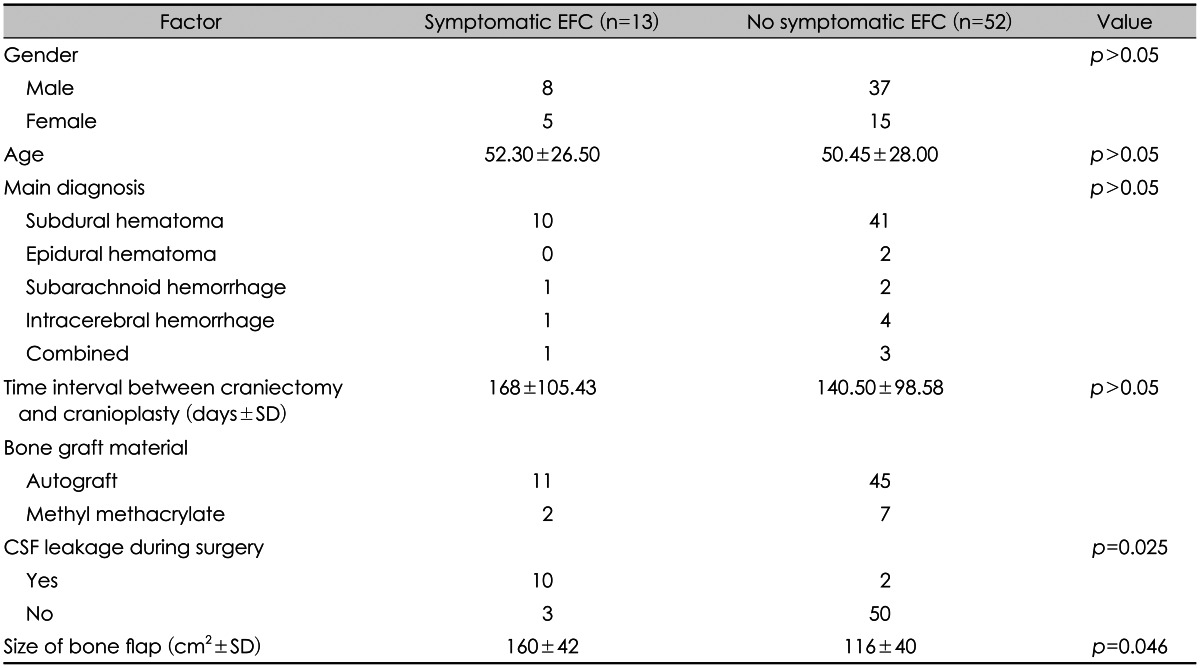
In the univariate analysis, the size of the bone flap (p=0.046) and CSF leakage during cranioplasty (p=0.025) were y significant decisive factors favoring the development of symptomatic EFC.
Other factors such as age, sex, initial diagnosis, time interval between craniectomy and cranioplasty, were not statistically significant (
Table 2).
TABLE 2
Comparison of clinical and radiographic data from patients with symptomatic (Group I) and asymptomatic epidural fluid collection (Group II)

Go to :

Discussion
Cranioplasty is a relatively commonplace neurosurgical practice, and is regarded as a simple and safe procedure. However, even with advanced neurosurgical techniques, a considerably higher complication rate compared to other elective cranial surgeries has been reported.
1011)
The representative complications include postoperative infections, hematoma, bone graft resorption, seizures, and fluid collection as a general risk of neurosurgery.
9)
It is well known that postoperative infection after cranioplasty is associated with significant morbidity, due to the need for removal of the bone flap, a course of long-term intravenous antibiotics, and its replacement at a later time.
5) However, reports of symptomatic EFC following cranioplasty are few, and limited to isolated case examples. Lee et al.
9) reported that EFC following cranioplasty was much more common than had previously been thought, but detectable mental changes or motor weakness caused by symptomatic EFC are relatively rare because they tend to regress or disappear over time. They insisted that both dural calcification and the presence of postoperative air bubbles could be predictive factors for the formation of EFC following cranioplasty. In the present study, an overall rate of symptomatic EFC following cranioplasty was about 15% more common than expected.
Although the exact cause of symptomatic EFC is still unknown, it has been hypothesized that it is not a single disease entity, but rather arises as a product of several complex factors.
The three potential causes posited thus far are as follows: first, dural stiffness caused by dural calcification, preventing expansion of the brain and causing an epidural 'dead space'. Second, an air bubble in the epidural space may initiate an inflammatory process, resulting in the formation of exudates.
29) Third, CSF may leak through the dura, possible due to injury caused during the cranioplasty, and lead to exudates from the dissected subgaleal region and muscle.
In our study, the factors regarding dural calcification were not studied because of the vague criteria defining this phenomenon.
Moreover, the presence of an air bubble was not studied as a significant factor in the development of EFC. Most of our cranioplasty patients (70% in Group I, and 40% in Group II) presented with air bubbles, as determined by postoperative brain CT scan images. We believe this happens largely due to the use of a Gelform when performing the cranioplasty.
Instead, based on the present study, we suggest that CSF leakage during cranioplasty can be a useful factor for predicting the development of symptomatic EFC. CSF may leak during a cranioplasty, and postoperative exudates from the dissected subgaleal region and close muscle layer may accumulate due to failure of the brain to expand. Moreover, a larger skull defect increases the likelihood of dural injury during cranioplasty. The frequency of the symptomatic EFC was also higher in patients with a large skull defect in this study.
Amongst the 13 patients in Group I, 11 patients were suc-cessfully treated with a simple drainage procedure. However, in 2 patients who showed CSF leakage during cranioplasty, evacuation of EFC via burr hole drainage using a 5 L catheter did not prevent repetitive fluid collection, and the infected bone flap had to be removed. We believe that conclusive confirmation of the CSF leakage site, its re-pair, and subsequent massive irrigation could prevent the repeated EFC and the necessity of bone flap removal.
To the best of the authors' knowledge, there has been no study assessing the development of symptomatic EFC following cranioplasty for the patients of TBI. In spite of the limitations inherent in a retrospective analysis, this study enhances the understanding of symptomatic EFC as being a relatively common and important complication following cranioplasty.
Go to :

Conclusion
The possibility of symptomatic EFC should be considered in cases in which CSF leakage occurs during cranioplasty, as well as in those that feature large skull defects. Great efforts should be made to avoid this unwanted complication after cranioplasty.
Go to :







 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download