Abstract
Objective
The aim of this preliminary collaborative study was to assess the clinical characteristics, management, and outcome of epidural hematoma (EDH) based on the data collected and registered in the Korean Trauma Data Bank System (KTDBS).
Methods
Of 2,698 patients registered in the KTDBS between September 2010 and March 2014, 285 patients with EDH were analyzed. Twenty-three trauma centers participated in the study voluntarily to collect data. We subcategorized the patients into two groups with good and poor outcomes. Various clinical characteristics and the time intervals with regard to treatment course were investigated to determine the relationship between these parameters and the functional outcome.
Results
Of multiple parameters for this analysis, older age (p=0.0003), higher degree of brain injury (p<0.0001), cases of surgical EDH (p<0.0001), time interval from trauma to hospital before 6 hours, and the decreasing pattern of Glasgow Coma Scale (GCS) between and initial and final GCS were strongly associated with poor outcome. Use of prophylactic anticonvulsant did not affect the functional outcome. There was an interesting difference in the use of mannitol in treating EDH between the urban and rural regions (p<0.0001).
Go to : 
Epidural hematomas (EDH) comprise 2% of total traumatic brain injury (TBI) but its mortality rate is as high as 33%.18) This particular neurosurgical emergency has been known for a long time. Yet, the pathological dynamics of EDH is not fully understood. Its heterogeneous responses to the treatment in the different subgroups of populations require further international attention and investigation.1115) Korea has recently attempted to collect a large cohort of data relating to multiple aspects of TBI for the past few years. Fatality of EDH has also partly urged the actions to emphasize the importance of epidemiological monitoring for prevention, revisiting the safety laws, and the upgrading trauma centers to ultimately improve the facilities and systems for better functional outcomes. The evolving nature of EDH is especially observed dynamically at the acute settings of different types of trauma; hence, the authors thought that this specific disease deserves a spot light for national investigation. To our knowledge, though it is preliminary, this is the first report to attempt in evaluating the multi-center data registered in the Korean Trauma Data Bank System (KTDBS) to unravel the patterns of clinical course of EDH in Korea and to identify the significant parameters in determining the outcomes of EDH.
Go to : 
The aim of this project was to conduct an epidemiologic research on the epidemiology of TBI in Korea. The data were voluntarily submitted by 23 trauma centers of all levels of designation. The data sheet of the KTDBS was composed of 392 items, containing information regarding the characteristics and etiology of the injury, diagnosis, treatment, and complications with respect to the outcome of patients. Two thousand six hundred ninety-eight patients were added to the database over its duration of the project.
Of 2,698 cases of TBI patients, 377 case of EDH were retrieved from the database. Patients younger than 16 years and patients with field cardiac arrest were excluded. Thus, the final cases of 285 patients with EDH were evaluated for this study. We subcategorized the patients into two groups with good and poor outcome. The following clinical parameters were compared with respect to the final outcome: age, gender, presence of skull fracture, treatment plan, degree of brain injury, time interval from trauma to hospital, types of injury mechanisms, time interval from trauma to initial computed tomography (CT), treatment plan after the initial CT, time interval from initial CT to follow-up CT, the reasons for follow-up CT, use of mannitol, prophylactic use of prophylactic anticonvulsant, time taken to decide a surgical treatment, and types of surgery. The difference of Glasgow Coma Scale (GCS) between the GCS upon arrival of the hospital and the lowest GCS during the hospital stay were also analyzed. All patients underwent CT scanning during the course of treatment. Outcome of 6 months following injury by the Glasgow Outcome Scale (GOS) in which 'good outcome' was defined as a good recovery or a moderate disability, and 'poor outcome' was severe disability, vegetative state, or death.7)
Data are expressed according to the properties of the variable. Continuous variables are presented as mean and standard deviation. Categorical variables are presented as frequency and percentage. In order to compare two groups, we performed the two-sample t-test or chi-square test (Fisher's exact test) as appropriate. Logistic regression analysis was used to identify the factors to predict the poor outcome and the result were expressed as odds ratio (OR) with 95% confidence interval (CI). A p-value less than 0.05 was considered statistically significant and all statistical analyses were conducted using SAS 9.4 version (SAS Inc., Cary, NC, USA).
Go to : 
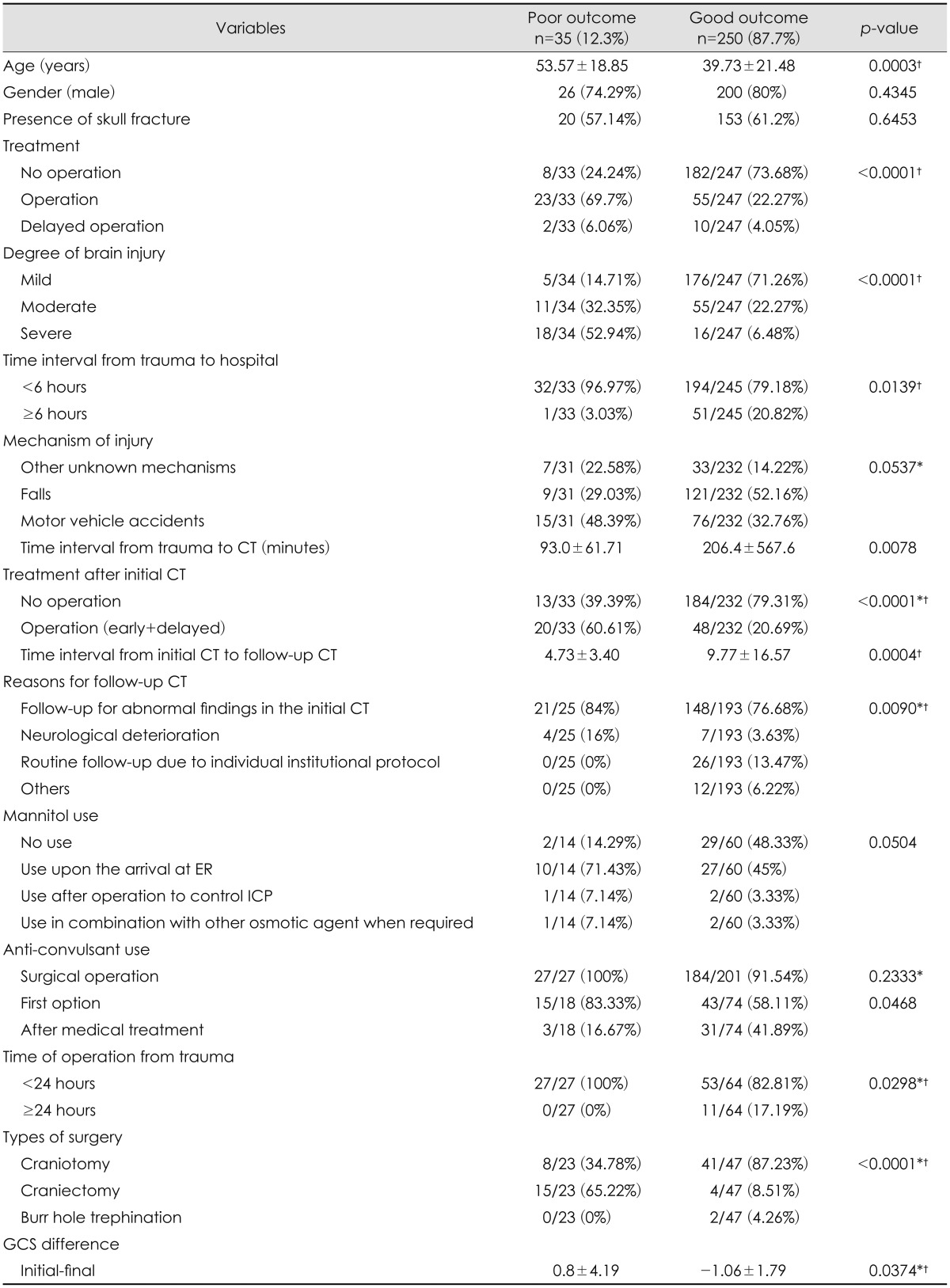
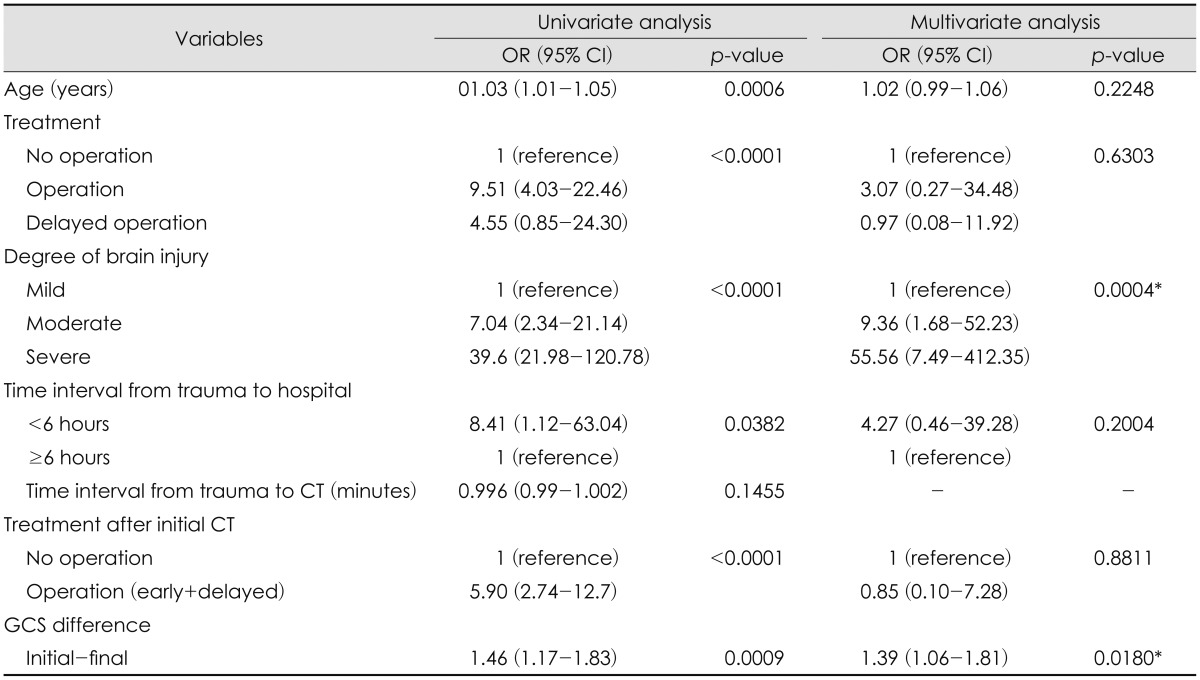
In order to identify the clinical factors associated with poor outcome, the study population was divided into two subgroups of good and poor outcome. The parameters of clinical characteristics were compared between these two groups. According to the demographic statistics of the patients, older age (p=0.0003), more severe the degree of brain injury (p<0.0001), cases of surgical EDH with craniectomy (p<0.0001), shorter time interval from trauma to hospital before 6 hours, shorter time interval from trauma to initial CT (p=0.0078) and the decreasing pattern of GCS between and initial and final GCS (p=0.0374) were strong predicting factors of poor outcome (Table 1). The injury mechanism of falls was more common in the patients with good outcome (121/232, 52.16%), however, it was not statistically significant (p=0.0537). Use of mannitol for controlling increased intracranial pressure (ICP) upon the arrival at emergency department was higher in the patients with poor outcome (10/14, 71.43%). On the other hand, those with good outcome received no treatment of mannitol for EDH (29/60, 48.33%). Again, the use mannitol was neither statistically significant (p=0.0504). According to the 1:1 univariate analysis, the odds of poor outcome for older patients were 1.03 times. Cases of surgical EDH showed the odd of poor outcome 9.51 times higher than those of non-surgical EDH. Compared to the patients with mild brain injury, those with moderate brain injury showed the odds of poor outcome 7.04 times higher than those with mild brain injury while those with severe brain injury showed the odds 39.6 times higher than those with mild brain injury. Shorter time interval from trauma to hospital before 6 hours showed the odds of poor outcome 8.41 times higher compared to that after 6 hours. Finally, the odds of poor outcome are shown 1.46 times higher when the patients' final GCS is lower than the initial GCS.
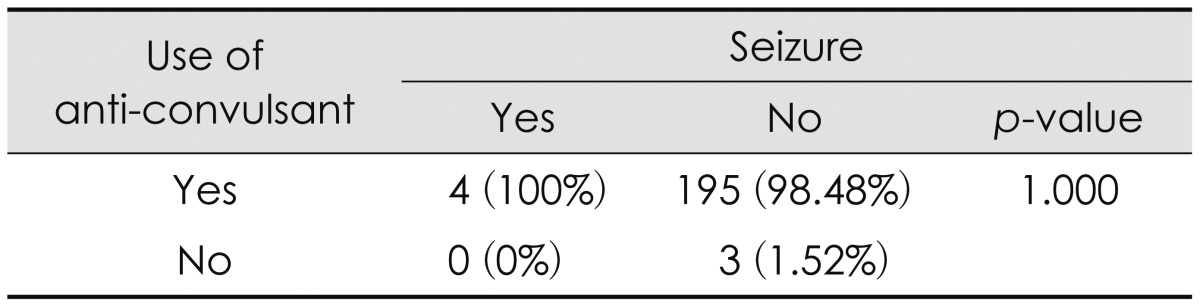
Clinical parameters associated with the poor outcome on the univariate analysis were entered into the multivariate model for further evaluation. The degree of brain injury (OR of 9.36 in moderate injury; 95% CI, 1.68-52.23; and OR of 55.56 in severe injury; 95% CI, 7.49-412.35, respectively; p=0.0004) and the GCS differences between the initial and final GCS (OR of 1.39; 95% CI, 1.06-1.81; p=0.0180) showed a statistically significant result as strong determining factors related to poor outcome (Table 2). Of note, prophylactic use of anticonvulsant did not affect the functional outcome (Table 3).
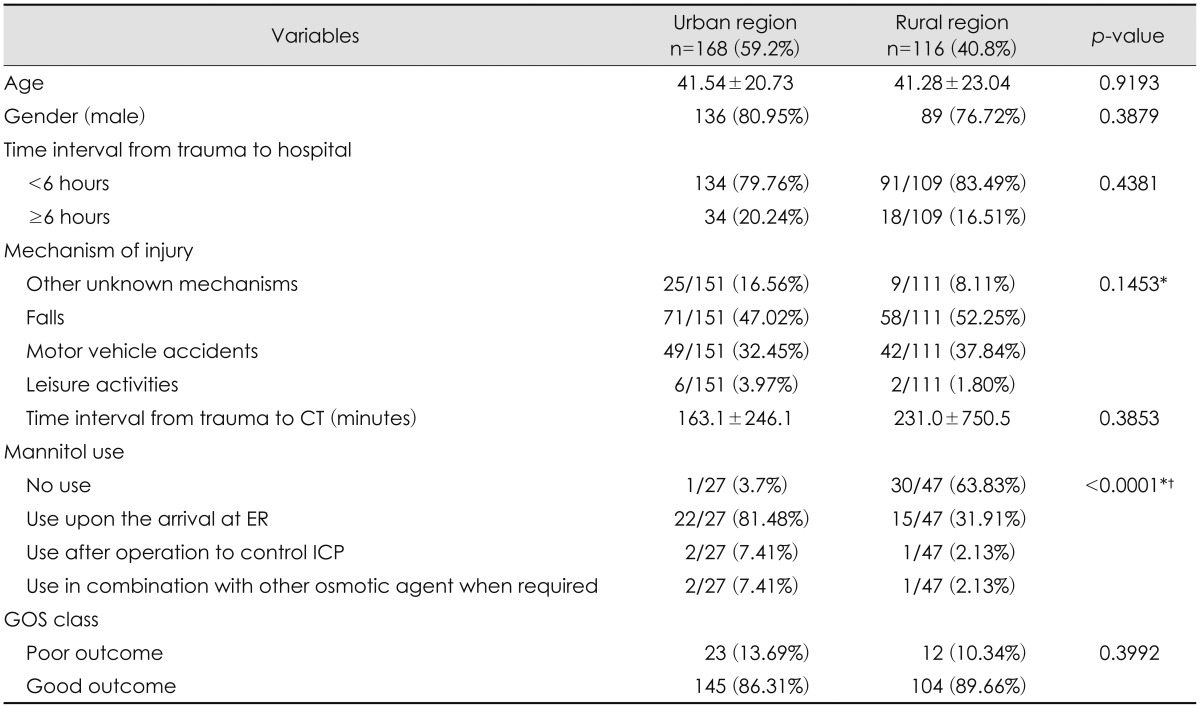
There was an interesting difference in the use of mannitol in treating EDH between the urban and rural regions [22/27 (81.48%) and 15/47 (31.91%), respectively; p<0.0001] (Table 4). There was 63.83% (30/47) of patients who received no mannitol treatment in the rural hospitals. This was a statistically significant difference between the two regions.
Go to : 
EDH is a complex entity of trauma in which the neurologic damage evolves after the impact.21828) Different mechanisms of injury with EDH are usually involved to create a diverse traumatic environment. In addition, different types of brain injuries result in multiple subdivisions of inconstant consequences.5)
As the population ages with longer life expectancy, trends of injury have also been changing with more injuries in the geriatric population.61524) As well as we have already witnessed in the analysis of our database, age is a major determinant of functional outcome.1524) For example, an injury mechanism of falls from heights is one of the leading causes of TBI in the elderly older than 65 years with a high risk of death.22) Even ground-level falls are risks to the elderly when it is generally and easily known to be a low risk in the younger population.19) Our data on EDH from KTDBS showed that falls are associated with good outcome of EDH (though it was not statistically significant). Nevertheless, this alarms us to collect more systematized data to confirm the difference, if there is any, in the domestic and international epidemiology of EDH. The results may even different in the types of brain injury other than EDH as well. It is imperative to be aware of the new injury types in the geriatric population.
As far as the degree of brain injury is concerned, one will immediately think of the pathological mechanism of early events of TBI, which is still much less understood though there is a great effort of basic research undertaken internationally to uncurtain the injury mechanisms at the cellular levels.827) However, macroscopically, the severity, duration of impact, duration without treatment, and nature of injuries determine the degree of brain injury. Degree of brain injury is often characterized by severe brain swelling, combination with traumatic subarachnoid hemorrhage, cerebral vasospasm, and delayed cerebral infarction after trauma.1826) EDH with additional intracranial injuries would act together in a simultaneous manner implicating to worsen the patients' condition. The accumulative damage will add on to escalate the extent of brain injury, which will eventually affect the functional outcome as we have already demonstrated in the analysis of our data. In the end, patients with severe GCS and the management of ICP determines the final outcome in patients with EDH as the response of recovery is dependent on the treatment effects during the clinical course and possibly on the patients' predisposing co-morbidity.5)
One must also bear in mind that there are also extrinsic factors which can potentially threaten the patients outside the hospital. Namely, the secondary injury by such as hypoxia or hypotension occurred, before the patient entered the emergency department due to lack of professional pre-hospital management, will be as critical as the impact of irreversible primary damage of neuronal pathways of consciousness at the first place.10)
A clinical deterioration of a patient with EDH is related with the size of EDH and its time-dependent enlargement. There was an analytical study reported that a CT scan performed less than 6 hours after the onset of trauma had an increased risk of hematoma enlargement.25) Our analysis of EDH data from KTDBS also confirms that the time interval from trauma to the initial CT scan before 6 hours is associated with a poor functional outcome. It is also reported that a series of CT scans in non-operative EDH patients should be obtained within 6 to 8 hours after the initial injury for better outcomes with prompt decision of treatment.20) Moreover, Ding et al.3) reported that the non-surgical patients with routine repeat CT scans have a better outcome than those with non-routine repeat CT. However, the same authors also mentioned that routine follow-up CT scans 48 hours after trauma may not also be required.316)
According to the guidelines of the Brain Trauma Foundation (BTF), the volume of EDH and the initial GCS are determinants of surgical indication of EDH. Not all patients benefit from the operation of EDH.21218) Our data showed that the poor outcomes are more associated with the cases of surgical EDH. Furthermore, the cases of decompressive craniectomy with hematoma evacuation of EDH are also associated with a poor outcome (p<0.0001). It is rather ironic that the act of surgery is not guaranteeing the functional outcome of the patients while the neurosurgeons have put so much effort and time to open the skull to relieve the increased ICP. Oppositely speaking, the patients undergoing the operation to decompress the ICP would have been already in a life-threatening condition due to severe brain injury that the surgery is the last resource for their survival. The surgery within 24 hours is indicative of a fast progression of the disease. Whereas the surgery 24 hours after trauma somewhat suggests a slow nature in the development of hematoma enlargement, henceforth, this is clinically associated with good outcome in our data analysis. Park et al.14) also reported that the ultra-early decompressive craniectomy for TBI did not improve the patient outcome after all. Surgery should not be the only solution of treating EDH.21218) At the same time, surgery should not be mandatory robotically even if the guidelines of BTF say so. There are number of clinical articles on the management of non-surgical EDH in the selected patients, such as elderly patients.21218) These data should be heavily considered in establishing more systematic and tailored protocols for treating EDH.121418)
GCS is a static measurement of consciousness of neurological patients. It may fluctuate from time to time and it may change dramatically early after injury. The proper assessment of initial GCS should be carried out ideally after hemodynamic and respiratory resuscitation of TBI patients. Nevertheless, under the same name of EDH, some patients would exhibit neurological deterioration meanwhile others would improve as time passes by. BTF mentions the importance of initial GCS at presentation. However, because of the unreliability of initial GCS in a certain group of patients, we retrieved the data from the KTDBS and attempted to calculate the difference between the initial and the final GCS in order to assess the functional outcome. As a result, we found that the larger the difference of GCS from the initial to the final GCS in a decreasing manner, the poorer the functional outcome of the patients.
Sufficient cerebral perfusion and prevention of increased ICP is a crucial tactic in managing TBI.23) Protocols of ICP management vary inevitably due to different experiences, and they vary even among neurosurgeons within the same institution. According to our data, mannitol seems to be sitting in the middle of controversy in the context of ICP management of EDH patients. Although mannitol was not directly associated with final outcome (Table 1), the individual institutional protocols in treating EDH and the time of mannitol given during the course of disease progression have not yet met a consensus in Korea (Table 4). If this is true, the effect of mannitol on the outcome of the patients with EDH must be scrutinized carefully in the near future. Implementation of evidence-based recommendations by BTF is related with the improvements in mortality in TBI.9) Adoption and integration of the basic guideline to individual institutional protocols is a step forward to treating a diverse pool of patients with EDH.421)
In hoping to be of some contribution for building a Korean prognostic model of EDH, we attempted to classify the clinical parameters in association with the functional outcomes by the analysis of data based on the KTDBS. By sharing the trauma data nation-wide and adopting the prognostic models, this national study will soon activate the improvement in the quality of neurosurgical care of EDH.25)
This study is a retrospective review of collected data from only 23 trauma centers in Korea, thus, these results may not yet reflect the whole EDH population in Korea. Non-operative groups of patients may be biased if the patient's family disagreed on the surgical treatment. A new prospective database needs to be more specified with more information and details regarding the decision making of treatments in the clinical practice. This data set did not include the predisposing co-morbidities and medical conditions of patients, thus, this will underestimate the differences in the course of coagulopathy of the individuals with EDH to a certain extent.17) This study has defined the clinical outcome as a functional outcome, not mortality, and this was assessed with the GOS. We may have to differentiate the severe EDH from mild EDH as the survival factors will be more relevant for severe EDH. The quality of health care systems have to be taken account in the analysis as it possibly determines the long-term outcome of the patients.25) In order to elucidate the variability of responses to EDH, a basic research on biomarkers and genetic components of the individual patients will be required in the analysis as well. For example, the recovery is poorer in patients with stroke or TBI who have the Apolipoprotein E-ɛ4 (APOE-ɛ4) allele than in those who do not have this allele.13) This is one of many examples of genomics partially playing a role in the complex course of disease.
Go to : 
The primary aim of the present study was to investigate how clinical parameters from the onset of trauma and during the treatment influence the patient outcome. This is the first report identifying the factors associated with poor and good outcomes of patients with EDH. A new strategy may be required to prevent patients being severely disabled for a long-term as it is eventually linked with the socio-economic problems in this country. The epidemiology of EDH is changing, therefore, the pre-hospital care, diagnostic instruments, critical care monitoring, and treatment have to be changed and validated continuously for better outcome. The degree of brain injury and the GCS difference were notable factors that were significant in determining the functional outcome of EDH. However, some issues have to be raised for further investigation as our current data is based mainly on the admission characteristics. The data from KTDBS will eventually serve as the basis for designing the optimal standards in the acute care of EDH and other TBI. In the end, the implementation of the revised guidelines fit for Korean trauma systems will be important in effectively preventing poor prognostic aspects of EDH.
Go to : 
References
1. Bae DH, Choi KS, Yi HJ, Chun HJ, Ko Y, Bak KH. Cerebral infarction after traumatic brain injury: incidence and risk factors. Korean J Neurotrauma. 2014; 10:35–40. PMID: 27169031.

2. Bezircioğlu H, Erşahin Y, Demirçivi F, Yurt I, Dönertaş K, Tektaş S. Nonoperative treatment of acute extradural hematomas: analysis of 80 cases. J Trauma. 1996; 41:696–698. PMID: 8858030.
3. Ding J, Yuan F, Guo Y, Chen SW, Gao WW, Wang G, et al. A prospective clinical study of routine repeat computed tomography (CT) after traumatic brain injury (TBI). Brain Inj. 2012; 26:1211–1216. PMID: 22571813.

5. Heinzelmann M, Platz A, Imhof HG. Outcome after acute extradural haematoma, influence of additional injuries and neurological complications in the ICU. Injury. 1996; 27:345–349. PMID: 8763290.

6. Herou E, Romner B, Tomasevic G. Acute traumatic brain injury: mortality in the elderly. World Neurosurg. 2015; 83:996–1001. PMID: 25731794.

7. Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975; 1:480–484. PMID: 46957.
8. Kawamata T, Katayama Y. Cerebral contusion: a role model for lesion progression. Prog Brain Res. 2007; 161:235–241. PMID: 17618981.

9. Keris V, Lavendelis E, Macane I. Association between implementation of clinical practice guidelines and outcome for traumatic brain injury. World J Surg. 2007; 31:1352–1355. PMID: 17464541.

10. Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008; 7:728–741. PMID: 18635021.

11. Nakamura N, Yamaura A, Shigemori M, Ono J, Kawamata T, Sakamoto T. Epidemiology, prevention and countermeasures against severe traumatic brain injury in Japan and abroad. Neurol Res. 2002; 24:45–53. PMID: 11783753.

12. Offner PJ, Pham B, Hawkes A. Nonoperative management of acute epidural hematomas: a "no-brainer". Am J Surg. 2006; 192:801–805. PMID: 17161097.

13. Padgett CR, Summers MJ, Skilbeck CE. Is APOE epsilon4 associated with poorer cognitive outcome following traumatic brain injury? A meta-analysis. Neuropsychology. 2016; 30:775–790. PMID: 26986748.
14. Park JH, Park JE, Kim SH, Lim YC, You NK, Ahn YH, et al. Outcomes of ultra-early decompressive craniectomy after severe traumatic brain injury-treatment outcomes after severe TBI. Korean J Neurotrauma. 2014; 10:112–118. PMID: 27169044.

15. Shimoda K, Maeda T, Tado M, Yoshino A, Katayama Y, Bullock MR. Outcome and surgical management for geriatric traumatic brain injury: analysis of 888 cases registered in the Japan Neurotrauma Data Bank. World Neurosurg. 2014; 82:1300–1306. PMID: 25128777.

16. Shin DS, Hwang SC, Kim BT, Jeong JH, Im SB, Shin WH. Serial brain CT scans in severe head injury without intracranial pressure monitoring. Korean J Neurotrauma. 2014; 10:26–30. PMID: 27169029.

17. Shoko T, Shiraishi A, Kaji M, Otomo Y. Effect of pre-existing medical conditions on in-hospital mortality: analysis of 20,257 trauma patients in Japan. J Am Coll Surg. 2010; 211:338–346. PMID: 20800190.

18. Soon WC, Marcus H, Wilson M. Traumatic acute extradural haematoma - Indications for surgery revisited. Br J Neurosurg. 2016; 30:233–234. PMID: 26742836.

19. Spaniolas K, Cheng JD, Gestring ML, Sangosanya A, Stassen NA, Bankey PE. Ground level falls are associated with significant mortality in elderly patients. J Trauma. 2010; 69:821–825. PMID: 20938268.

20. Sullivan TP, Jarvik JG, Cohen WA. Follow-up of conservatively managed epidural hematomas: implications for timing of repeat CT. AJNR Am J Neuroradiol. 1999; 20:107–113. PMID: 9974064.
21. Tarapore PE, Vassar MJ, Cooper S, Lay T, Galletly J, Manley GT, et al. Establishing a traumatic brain injury program of care: benchmarking outcomes after institutional adoption of evidence-based guidelines. J Neurotrauma. 2016; [epub ahead of print Apr 29, 2016.]. DOI: 10.1089/neu.2015.4114.

22. Thompson HJ, McCormick WC, Kagan SH. Traumatic brain injury in older adults: epidemiology, outcomes, and future implications. J Am Geriatr Soc. 2006; 54:1590–1595. PMID: 17038079.

23. Thompson HJ, Rivara FP, Jurkovich GJ, Wang J, Nathens AB, MacKenzie EJ. Evaluation of the effect of intensity of care on mortality after traumatic brain injury. Crit Care Med. 2008; 36:282–290. PMID: 18007264.

24. Tokutomi T, Miyagi T, Ogawa T, Ono J, Kawamata T, Sakamoto T, et al. Age-associated increases in poor outcomes after traumatic brain injury: a report from the Japan Neurotrauma Data Bank. J Neurotrauma. 2008; 25:1407–1414. PMID: 19086809.

25. von Steinbüchel N, Wilson L, Gibbons H, Hawthorne G, Höfer S, Schmidt S, et al. Quality of Life after Brain Injury (QOLIBRI): scale development and metric properties. J Neurotrauma. 2010; 27:1167–1185. PMID: 20486801.

26. Wong GK, Yeung JH, Graham CA, Zhu XL, Rainer TH, Poon WS. Neurological outcome in patients with traumatic brain injury and its relationship with computed tomography patterns of traumatic subarachnoid hemorrhage. J Neurosurg. 2011; 114:1510–1515. PMID: 21332291.

27. Yokobori S, Nakae R, Yokota H, Spurlock MS, Mondello S, Gajavelli S, et al. Subdural hematoma decompression model: A model of traumatic brain injury with ischemic-reperfusional pathophysiology: A review of the literature. Behav Brain Res. [epub ahead of print May 25, 2016.]. DOI: 10.1016/j.bbr.2016.05.055.

28. Zakaria Z, Kaliaperumal C, Kaar G, O'Sullivan M, Marks C. Extradural haematoma-to evacuate or not? Revisiting treatment guidelines. Clin Neurol Neurosurg. 2013; 115:1201–1205. PMID: 23759341.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download