Abstract
The management guideline for traumatic brain injury (TBI) recommends high-dose barbiturate therapy to control increased intracranial pressure refractory to other therapeutic options. High-dose barbiturate therapy, however, may cause many severe side effects; the commonly recognized ones include hypotension, immunosuppression, hepatic dysfunction, renal dysfunction, and prolonged decrease of cortical activity. Meanwhile, dyskalemia remains relatively uncommon. In this study, we report the case of a hypokalemic patient with severe rebound hyperkalemia, which occurred as a result of barbiturate coma therapy administered for TBI treatment.
Go to : 
Dyskalemia (hypokalemia and/or rebound hyperkalemia) is a rare but a dire complication of high-dose barbiturate coma therapy in order to reduce intracranial pressure (ICP) in traumatic brain injury patients; it can be easily overlooked due to its low rate of incidence. In this report, the authors summarized the clinical course of a patient who developed hypokalemia with severe rebound hyperkalemia as a result of therapeutic barbiturate coma therapy during the treatment of traumatic head injury.
Go to : 
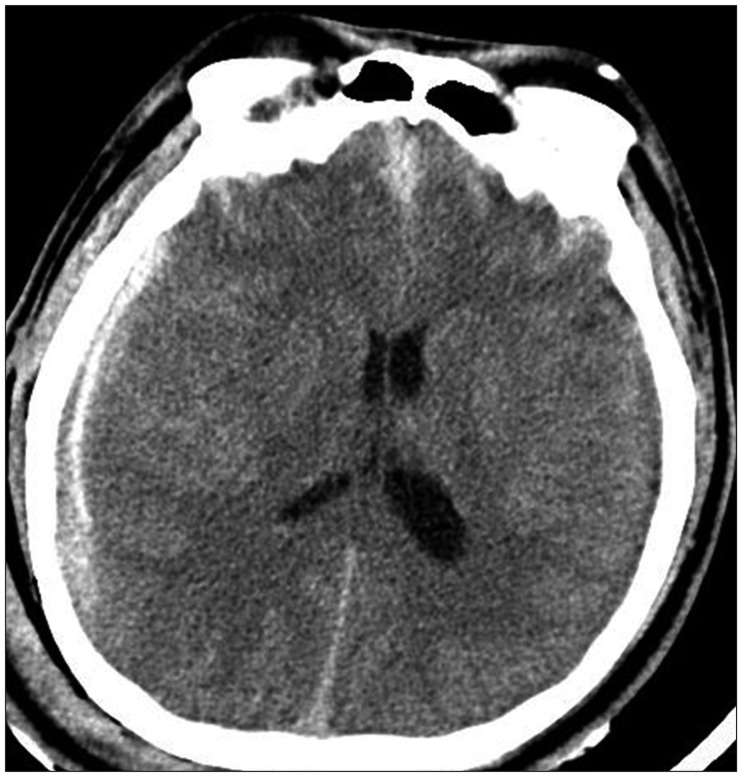
A 30-year-old male without any pre-existing medical history was admitted to the emergency room at our hospital after a motor vehicle crash. On neurologic examination, the patient had an initial Glasgow Coma Scale of 4, requiring endotracheal intubation to keep airway. Owing to diffuse traumatic subarachnoid hemorrhage and traumatic subdural hematoma on the right cerebral hemisphere (Figure 1), the cerebral perfusion pressure (CPP) of the patient was less than 50 mmHg, in spite of several efforts to reduce the ICP. Thus, a decompressive craniectomy was performed to reduce ICP. After the operation, a barbiturate coma therapy was induced with a thiopental infusion. A loading dose of thiopentone 600 mg (10 mg/kg) was given, followed by a maintenance dose of 120 mg/h (2 mg/kg/h). Intensive care management included mechanical ventilation to keep normocapnea, maintenance of normoglycemia, normothermia and blood pressure support to keep CPP above 70 mmHg. Twenty hours later, the serum potassium level started to fall. The patient was given approximately 120 mmoL of potassium in order to maintain the serum potassium level within a normal range. However, the serum potassium level kept dropping to 2.1 mmol/L for next twenty-four hours. Another ten hours later, the lowest potassium level of 1.7 mmol/L was documented, which required administration of epinephrine and atropine to deal with critical arrhythmias (ventricular tachycardia, atrial fibrillation). For 72 hours during barbiturate coma therapy, he had been given approximately 680 mmoL of potassium; a serum level of 2.8 mmol/L was achieved. At the point the potassium infusion was stopped and the arrhythmias disappeared. Seventeen hours after cessation of the thiopental infusion, the patient suddenly developed left bundle branch block, which progressed to pulseless ventricular tachycardia. The potassium levels were 8.9 mmol/L, later increasing to 9.7 mmol/L. This hyperkalemia was treated with calcium chloride, furosemide, insulin in 50% dextrose and cation exchange (Resonium®) enemas. The serum potassium level dropped down to 6.2 mmol/L, finally normalizing after thirty-four hours. The biochemical disturbance subsequently stabilized. However, unfortunately, the patient was confirmed brain death on 10th day of admission.
Go to : 
Barbiturates have many beneficial effects for the injured brain. Marshall et al. asserted in their report of 100 consecutive patients with intractable intracranial hypertension who treated with high-dose barbiturate infusion, barbiturates are useful in uncontrolled intracranial hypertension. Eisenbuerg et al.5) suggested that the use of high-dose barbiturates has been shown to control ICP and may reduce mortality in a small group of patients. Cormio et al.2) also reported that barbiturate coma can be a useful method for reducing ICP in selected patients. However, in cases of patients with overwhelmingly severe injuries are not likely to benefit from barbiturate coma. Barbiturate may reduce the cerebral metabolic rate.37) Barbiturates may exert protective effects include stabilization of lysosomal membranes, modification of amino acid and neurotransmitter release, alteration of fatty acid metabolism, suppression of hyperactivity induced by catecholamine, membrane stabilization suppression of seizures and so on.178)
Barbiturates may also have adverse effects. Majdan et al.6) reported that high-dose barbiturate treatment not only lowers ICP but it causes hemodynamic instability, leading to lowering of the mean arterial pressure below 70 mmHg. Cruz's4) report had observed that 32% patients given barbiturates for control of increased ICP had a reduction in jugular venous oxygen saturation to less than 45%. This finding was associated with a poor outcome. Commonly recognized side effects of barbiturate infusion include hepatic and renal dysfunction, systemic hypotension, and immunosuppression. Relatively less well-recognized complication is that of severe potassium disturbance. There may be multiple mechanisms of hypokalemia during the high-dose barbiturate therapy. First, it is related to sympathetic stress response that results from the head trauma and a catecholamine surge. In addition, hypokalemia can be developed during the course of treatments such as the use of mannitol, vasopressor therapy, or insulin infusion. Second, barbiturate causes decrease of intracellular lactate and increase of pyruvate production, due to the inhibition of phosphofructokinase, which, in turn, leads to an increase in intracellular pH and potassium concentration. Barbiturate also inhibits voltage-dependent potassium currents, resulting in intracellular sequestration of potassium. Rebound hyperkalemia is difficult to explain. There are no satisfactory explanations for rapid developed rebound hyperkalemia; however, the intracellular accumulation of potassium during potassium replacement to correct hypokalemia is suggested to be one of the major players.
Go to : 
Dyskalemia may be associated with initiation and cessation of barbiturate therapy. Therefore, a close monitoring of plasma biochemistry is required. As shown in this case, during the barbiturate coma therapy, life-threating hypokalemia and rebound hyperkalemia can occur in the same patient. Most text books recommend the maximum potassium infusion rate as 20 to 40 mmol/h. But it may not be sufficient in some patients with extreme hypokalemia. On the other hand, using massive amounts of exogenous potassium to correct severe hypokalemia can induce life-threating hyperkalemia. This is the dilemma. Neurosurgeons should be aware of this and vigilant in monitoring the serum potassium during barbiturate therapy. We suggest that asymptomatic hypokalemia should be corrected less aggressively and a designed tapering cessation of barbiturate should be considered over abrupt cessation.
Go to : 
References
1. Cai Z, McCaslin PP. Acute, chronic and differential effects of several anesthetic barbiturates on glutamate receptor activation in neuronal culture. Brain Res. 1993; 611:181–186. PMID: 8334512.

2. Cormio M, Gopinath SP, Valadka A, Robertson CS. Cerebral hemodynamic effects of pentobarbital coma in head-injured patients. J Neurotrauma. 1999; 16:927–936. PMID: 10547101.

3. Crane PD, Braun LD, Cornford EM, Cremer JE, Glass JM, Oldendorf WH. Dose dependent reduction of glucose utilization by pentobarbital in rat brain. Stroke. 1978; 9:12–18. PMID: 622738.

4. Cruz J. Adverse effects of pentobarbital on cerebral venous oxygenation of comatose patients with acute traumatic brain swelling: relationship to outcome. J Neurosurg. 1996; 85:758–761. PMID: 8893711.

5. Eisenberg HM, Frankowski RF, Contant CF, Marshall LF, Walker MD. High-dose barbiturate control of elevated intracranial pressure in patients with severe head injury. J Neurosurg. 1988; 69:15–23. PMID: 3288723.

6. Majdan M, Mauritz W, Wilbacher I, Brazinova A, Rusnak M, Leitgeb J. Barbiturates use and its effects in patients with severe traumatic brain injury in five European countries. J Neurotrauma. 2013; 30:23–29. PMID: 22950895.

7. Michenfelder JD. The interdependency of cerebral functional and metabolic effects following massive doses of thiopental in the dog. Anesthesiology. 1974; 41:231–236. PMID: 4854910.

8. Morgan WW, Bermudez J, Chang XY. The relative potency of pentobarbital in suppressing the kainic acid- or the N-methyl-D-aspartic acid-induced enhancement of cGMP in cerebellar cells. Eur J Pharmacol. 1991; 204:335–338. PMID: 1663461.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download