Abstract
Objective
The optimal threshold of the infusion volume of cement has been a continuous subject in percutaneous vertebroplasty (PVP). This study verifies a causal relationship between the cement volume and the clinical outcome, and suggests the parameters of the appropriate volume of cement in PVP.
Methods
This is a retrospective study. One hundred nine patients, who underwent PVP between 2012 and 2015, were included in the study. Various factors such as patients' fracture levels, fracture types, fracture body volumes, fracture rates, bone mineral densities, and infused cement volumes were analyzed. Cement infusion ratios were calculated, using total amount of infused cement and fractured body volume. Follow up was done after one-week, one-month and three-months, postoperatively. Changes in the middle body height and the cement leakage levels were monitored and clinical outcomes were evaluated using a visual analogue scale.
Results
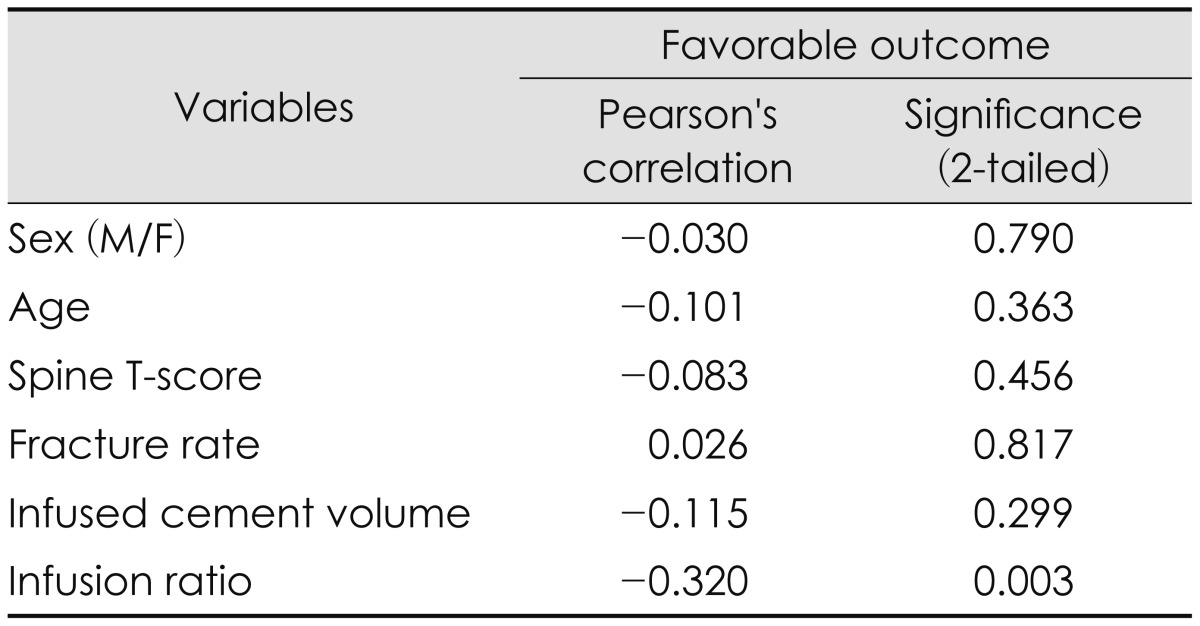
Among the variables, the infusion ratio (r=-0.320, p=0.003, Pearson's correlation) was the only index that showed a significant cause and effect relationship with favorable clinical outcome, except the group with a T-score of higher than -2.5, and the group with a upper thoracic vertebral level. The patients with a cement infusion ratio of 27.8% or more of the fractured body volume had favorable results.
Go to : 
The etiology of osteoporotic vertebral compression fracture is multifactorial.23) Osteoporosis is the most common metabolic disorder of the bone and in the case of elderly osteoporotic compression fracture of vertebrae, patients often have risk factors for surgery under general anesthesia10) If fractured vertebrae are not stabilized early, compression may progress, resulting in persistent back or neurologic deterioration due to kyphosis. Age-related deformity and secondary medical problems caused by restricted movements may additionally develop.19) For the treatment of symptomatic osteoporotic compression fractures, percutaneous vertebroplasty (PVP) is one of the most effective minimally invasive procedures and has become one of the most rapidly developing techniques in spinal surgery.11)
The goal of PVP is to provide immediate pain relief and bone strengthening in symptomatic vertebral compression fractures.7) Despite some debate, the stabilization of micro-movement, delay of progression of compression, and the thermal and chemical neural damage have been suggested as possible mechanisms of pain relief after PVP.3561521) There are many studies done on appropriate cement volume in PVP, however, the correlation between the cement volume and clinical outcome has still been under debate.413161722) It has been reported that a higher cement volume improves the results of mechanical restoration of vertebrae by enhancing strength and stiffness in PVP. In a recent cadaver study, PVP increases compression resistant strength and intradiscal pressure when cement volume was infused in at least 15% of the fractured vertebral volume.20) On the other hand, according to another study, cement leakage could be aggravated by excessive amount of cement infusion into the fractured vertebrae.27)
The purpose of this study is to verify a causal relationship between the infused cement volume and the clinical outcome, and suggests the parameters of the appropriate volume of cement in PVP by analyzing cement volume, level of fractured vertebrae, type and grade of fractured vertebrae, bone mineral density, and clinical outcome in patients with osteoporotic compression fracture.
Go to : 
One hundred nine among two hundred ninety three patients who underwent PVP for thoracolumbar compression fracture in the authors' hospital, between 2012 and 2015, were retrospectively analyzed. Those who had pathologic fractures or fractures in more than two vertebral segments were excluded from the study. Three-month follow-up data were analyzed and those who had been lost from the follow up were excluded from this study, as well.
All patients underwent two-weeks of absolute bed rest with conservative treatments prior to PVP except those who were older than 80 years at the time of admission or had medical problems, such as pneumonia. The patients who had -2.5 or lower T-score of any of lumbar or femur in bone mineral density (BMD) received the procedure. The patients were examined for tenderness and motion-induced pain in the fractured site before and after conservative treatment and the procedure. computed tomography (CT) and magnetic resonance imaging (MRI) scans were conducted for the definite diagnosis of fractures. With plain X-rays, original body heights of compressed vertebrae were estimated by measuring the mean heights of two adjacent vertebrae. If the adjacent vertebrae were fractured previously, the nearest vertebrae free from injury were used for estimation. Dual-energy X-ray absorptiometry (DXA; Hologic Inc., Waltham, MA, USA) scans were conducted in the femur neck areas as well as lumbar spine to measure the T-score of the BMD. Yet, only the T-scores of the lumbar spines were included for analysis in order to evaluate the correlation between T-scores of the spine and the clinical results. Moreover, as reference T-scores, the median values were selected, excluding the highest and lowest values among the lumbar T-scores. Thus, some patients were osteopenic, not osteoporotic, according to the analysis data.
Before the procedures, formal informed consents were taken from all patients and the study was executed in compliance with the Helsinki Declaration.
PVP was performed under C-arm monoplane or biplane fluoroscopy. During the procedure, the patient was carefully monitored with electrocardiogram, sphygmomanometer, and pulse oximeter. PVP was performed by three experienced neurosurgeons using the same protocol. Under local anesthesia, the Jamshidi's needle was inserted into the vertebra through a bilateral pedicle approach. The poly (methyl methacrylate) (PMMA) cement was then infused slowly into one side at a time to prevent cement leakage. When a cement leakage was detected lateral or anterior to the vertebrae, cement infusion was discontinued temporarily and carefully resumed only when no additional leakage was confirmed. When additional leakage was observed, cement infusion was suspended. When leakage into the vertebral foramen was suspected, cement infusion was halted immediately, the patient was examined neurologically, and the extent of canal encroachment was verified with CT scan, postoperatively. The patients started ambulation with braces on, six hours after the procedure. Further compression after the procedure was checked with a standing view of plain spine X-rays.
When cement leakage occurred, the type of the leakage was categorized with an X-ray or CT scan, according to the method by Yeom et al.28) The semi-quantitative visual grading of vertebral fracture was determined by the classification of Genant et al.8) Intervertebral vacuum cleft (IVVC) and preexisting adjacent fractures were identified. The 'infusion ratio' was calculated by dividing the infused cement volume by the fractured body volume. The fractured body volume, the compression rate, the compression progression rate, and the amount of cement leakage were measured with X-ray and CT scans using the PiViewSTAR (INFINITT; Infinitt Healthcare, Seoul, Korea) Dicom viewer. Volumetric values were obtained by accumulation of the width of vertebral bodies in 3 mm-thick sliced images, which were calculated and interpreted by one technician.
The pain was scored using the visual analogue scale (VAS) score from zero to ten. Patients were followed up on first week, after one-month, and three-months, postoperatively. Patients who had a VAS score less than three were classified as 'favorable outcome' group whereas three or higher as 'unfavorable outcome' group.
T-tests, ANOVA, χ2 tests, Fisher's exact tests, and bivariate correlation analyses were conducted on multiple variables such as volumetric data, outcome, age, sex, and the T-score. Simple linear regression analyses were applied to sequent variables. The mean and the mean dispersion of the measured variables were verified, and their 95% confidence interval was confirmed. Multiple linear regression analyses were applied to the factors that affect the cement infusion ratio and the volumetric data. The receiver-operating characteristic (ROC) curve and the area under the curve (AUC) were measured to confirm the correlation between the infusion ratio and the clinical outcome of PVP. The cut-off value was determined based on the maximum AUC value as having high sensitivity and specificity. Statistical significance was accepted when the p-value was less than 0.05 (SPSS statistical software 19.0, SPSS Inc., Chicago, IL, USA).
Go to : 
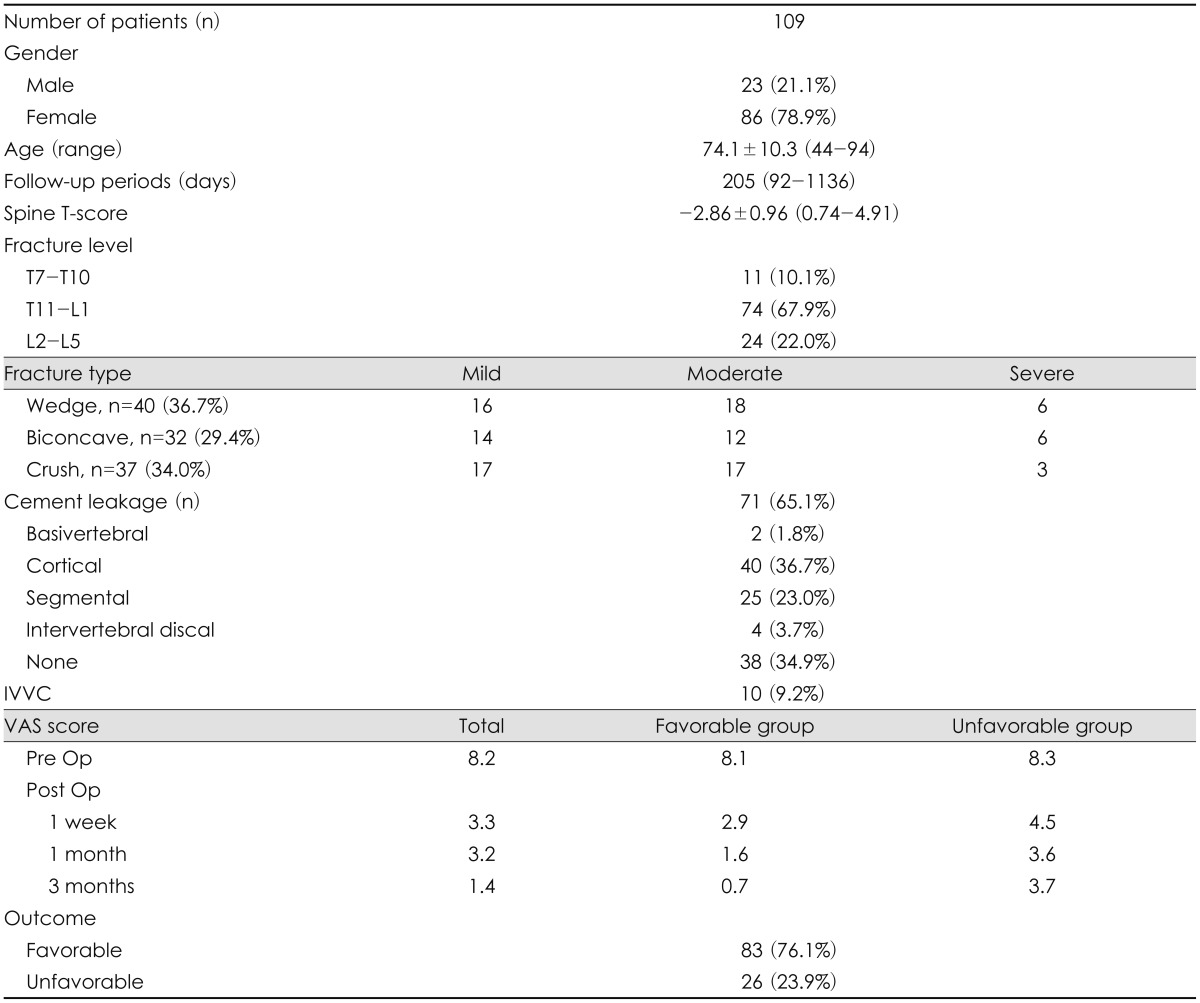
The patient demographics are summarized on Table 1. The mean age of the 109 patients included in the study was 74.1±10.3 (range, 44-94), and 86 of them (78.9%) were female. The mean follow-up period was 205 days (range, 92-1,136). The mean BMD was 0.71±0.15 g/cm2 (range, 0.28-1.30), and the mean T-score was -2.86±0.96 (range, 0.74-4.91). The number of patients with a spinal T-score of lower than -2.5 was 74 (67.9%). Fracture level was categorized as upper thoracic (T7-10), thoracolumbar (T11-L1), and lumbar (L2-5). Thoracolumbar injury was predominant above all, 74 cases (67.9%). Based on the fracture classification suggested by Genant et al.8), 40 cases of wedge type (36.7%), 37 cases of crush type (34.0%) and 32 cases of biconcave type (29.7%) were verified. Cement leakage occurred in 71 patients (65.1%). Most cases were of the cortical and segmental type, only 2 cases (1.8%) were basivertebral type, according to the classification by Yeom et al.28) IVVC exist in 10 patients (9.2%). The mean fracture rate of female patients was 12.58±11.63% (range, 10.1-15.17), whereas that of male patients was 11.88±9.28% (range, 7.9-15.9). The mean infusion ratio of each female and male patient was 24.87±7.51% (range, 23.7-26.5), and 3.18±5.91% (range, 20.6-25.7). The number of patients who showed favorable outcome was 83 (76.1%) while unfavorable outcome was 26 (23.9%). Their mean VAS of preoperative, one-week, one-month and three months postoperatively were 8.1, 2.9, 1.6, and 0.7, respectively, in the favorable prognosis group. The values were 8.3, 4.5, 3.6 and 3.7, respectively, in the unfavorable prognosis group (Table 1).
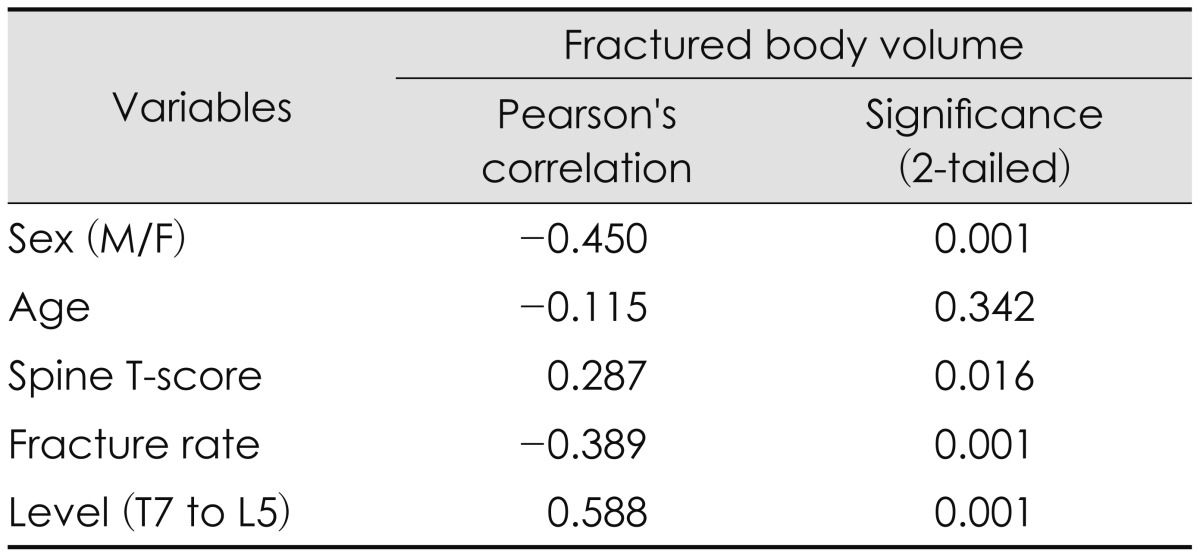
Among the favorable outcome group, bivariate correlate analyses were conducted on continuous variables such as age, T-score, fracture rate, intervertebral cement volume, as well as the categorical variables such as gender and vertebral level. The fractured vertebral body volume had correlations with sex, T-score, fracture rate, and fracture level, except for patient's age (Table 2).
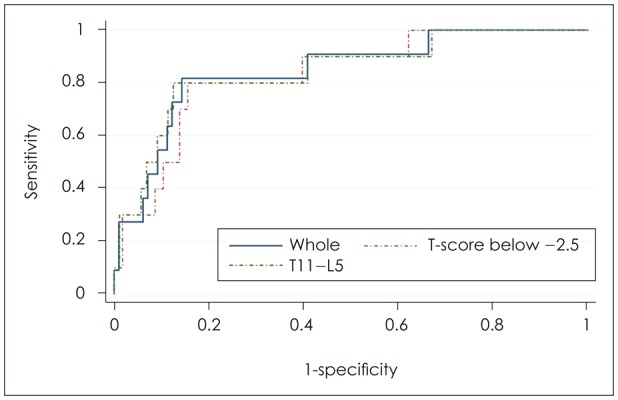
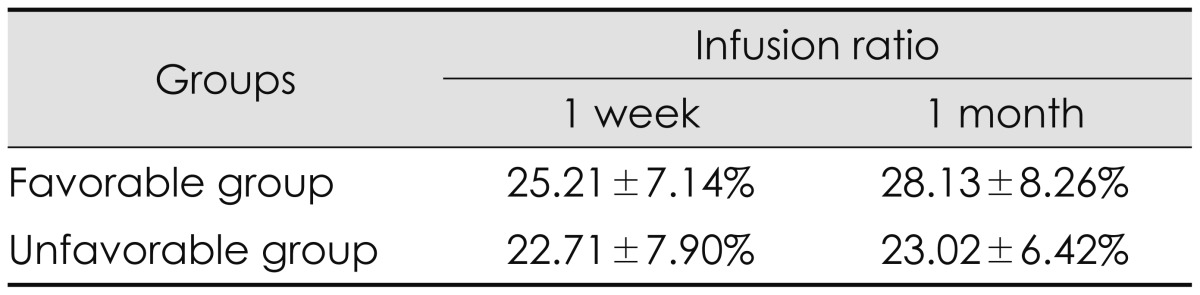
The mean infusion ratio (%) of all patients was 24.61±7.40%. The mean infusion ratio of the favorable group was 25.21±7.14%, and that of the unfavorable group was 22.71±7.90% in the first week follow up. The mean difference was 2.50%. Interestingly, the mean infusion ratio of the favorable group after a month postoperatively was 28.13±8.26%, and that of the unfavorable group was 23.02±6.42% showing a mean difference of 5.11%. In accordance with the follow-up period, the infusion ratio of favorable outcomes had changed (Table 3). Bivariate analyses were conducted on the various factors that might affect the outcome of PVP in the whole group and the favorable outcome group. The infusion ratio (r=-0.320, p=0.003, Pearson's correlation) was the only index that showed a significant cause and effect relationship with favorable clinical outcome (Table 4). The results of the linear regression analyses on the infusion ratio and outcomes were also statistically significant ANOVA (p<0.001). The maximum ROC AUC value of the infusion ratio and the outcome was 0.85 at a 27.83% infusion ratio with sensitivity and specificity 81.8% and 85.7%, respectively (Figure 1).
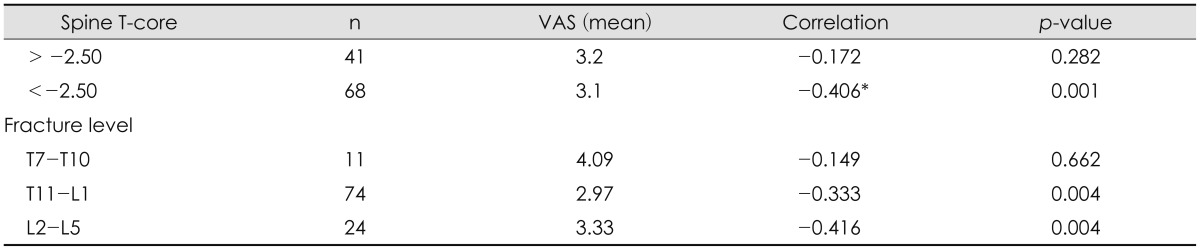
In the spinal T-score comparison, the 'normal to osteoporotic' group, with a T-score of higher than -2.5 did not demonstrate a significant correlation between infusion ratio and their outcomes (p=0.282). In the comparison by compressed vertebral levels, no significant correlation between infusion ratio and their outcomes was observed in the upper thoracic level group (p=0.662). Whereas in the comparison between the group with a T-score of lower than -2.5, and the group with a T11 or lower vertebral level, a significant correlation was observed between infusion ratio and their outcomes (Table 5). The maximum AUC value in the -2.5 or lower T-score group and the T11-L5 group were 0.83 and 0.84 respectively, with infusion ratio of 27.83% (80.0% sensitivity and 84.5% specificity) and 28.64% (80.0% sensitivity and 87.5% specificity), showing no significant differences. Therefore, an infusion ratio of at least 27.83% may be required to obtain favorable outcomes (Figure 1).
The correlation between infusion ratio of the cement and leakage type was analyzed. According to the leakage criteria suggested by Yeom et al.28), the cortical defect leakage type was observed in 40 patients (36.8%), the segmental vein leakage type in 25 patients (23.0%), the basivertebral vein leakage type in 2 patients (1.8%), and the intradiscal leakage type in 4 patients (3.7%). Logistic regression analyses were conducted on each type of cement leakage, which did not show any significant correlation with the infusion ratio (p=0.152).
Go to : 
PVP is a simple, noninvasive, low-risk procedure that provides immediate and durable pain relief and functional improvement to patients with osteoporotic vertebral compression fractures.126) Although its simplicity and safety, clinical success of PVP is another issue, influenced by the injected volume of PMMA and BMD.2) Based upon the hypothesis that vertebral stability is determined by the cement infusion volume, sufficient cement infusion is crucial in PVP. In this study, the authors focused on verifying an optimal intervertebral cement volume for favorable outcomes.
There are multiple studies, which investigated the correlation between the intravertebral cement volume and the clinical outcome through in vitro, in vivo and experimental studies.912131416171824) However, the consensus has not yet been established, since the optimal cut-off value varies according to each study. Al-Ali et al.1) and Kaufmann et al.13) asserted that there was no significant correlation between the infused cement volume and clinical outcomes. However, their mean cement volume ranged from 3.0 to 3.4 mL, which was a small amount, and they analyzed the infused cement volume only, not considering the severity of the fracture. Hence, the scientific evidence has been still insufficient to contend how infused cement volume is important to obtain successful outcome in vertebroplasty.
According to a cadaveric study of Liebschner et al.16), the minimal cement infusion ratio to recover the physical strength of compressed vertebrae was 15%. Kim et al.14), on the other hand, suggested 30% of infusion ratio for recovering the normal strength level of compressed vertebrae through experimental simulation study. Notwithstanding the difference of value, many studies assert that cement infusion ratio contributes to the recovery of the mechanical strength and stiffness.914161718) If the optimal amount of cement is determined solely based on each surgeon's experience, however, there is high possibility of inconsistency since the amount of cement may vary depending on the vertebral level, in addition to the surgeon bias. Thus, it is important to review the patients' fracture status carefully and establish a solid plan of cement volume amount before the procedure. In order to facilitate the planning, measuring optimal cement infusion ratio is necessary as a reference value.
In the most recent in vivo study of Jin et al.12) on osteoporotic vertebral fracture, a minimal cement infusion ratio of 11.64% was suggested for reducing pain and minimizing complication developments after the procedure. They advocated a minimal cement infusion ratio to relieve pain, emphasizing the risk of complications as a consequence of massive cement infusion. On the other hand, Nieuwenhuijse et al.24) suggested that a higher cement infusion ratio of 24% is required to recover physical strength of the compressed vertebra and reduce pain. In this study, we suggested as high as a 27.8% infusion ratio as an effective ratio for favorable outcomes. The ratio was similar to that of Kim et al.14), whose in vitro study determined that a 30% infusion ratio was effective for strength and stiffness recovery. In our study, the mean infusion ratio of the favorable group was 25.21±7.14% in the first week follow up. After a month, however, the value became 28.13±8.26%, which implies that even the pain was relieved with less than 27.8% of infusion ratio, temporarily, and it ended up aggravating as time goes by. In other words, patients who underwent PVP with less than 27.8% of infusion ratio gradually went over from favorable outcome group to unfavorable outcome group.
Leakage of cement out of the vertebral body may be subclinical, but it also may bring on devastating complications such as pulmonary embolism, neurological, and additional fractures through the endplate.2527) In a study of the major complications of cement leakage by Ryu et al.27), the complication of the cement leakage was caused by a high infusion volume. The mean infusion ratio of the leakage group was slightly higher than that of the non-leakage group. However, in this study, significant correlation between the infusion ratio and cement leakage was not found on the linear regression analysis. The difference of the two studies are that, Ryu et al.27) compared the group using injector for cement infusion with the group of manual infusion, whereas, all patients underwent manual infusion in our study. Overall frequency of cement leakage was relatively higher in this study, however, there was no serious clinical complication such as symptomatic pulmonary embolism or procedure related paralysis. We believe that was because the operators abide by our protocol, strictly; slow manual cement infusion into one side at a time, temporary discontinuance of infusion on a lateral or anterior cement leakage, and cessation of cement infusion on suspicion of leakage into the vertebral foramen.
In a volumetric analysis on the associated factors, the osteopenia group showed no correlation between the cement infusion ratio and their clinical outcomes, whereas the group with a T-score of lower than -2.5 showed a significant correlation. This result means, in an osteopenic group, paying attention to the avoidance of complications is recommended rather than increasing the infusion ratio. Meanwhile, in an osteoporotic group, attainment of the infusion ratio up to 27.8% is recommended. A similar result was found in Graham's study of the correlation between the BMD and infusion ratio, patients with a low T-score required the use of a higher infusion volume for favorable outcomes.9) In the upper thoracic level, above the 11th thoracic spine, there was less correlation between the infusion ratio and clinical outcomes, comparing with the thoracolumbar junction and lumbar area. Thus, patients with fractures in the upper thoracic level should be managed focusing on avoiding complications.
The goal of this study is to seek an optimal infusion ratio through tailored methods to improve the clinical outcome. Previously, one study found an optimal infusion ratio by the use of a maximum AUC point, the other study found it using a high specificity point.12,24) In our study, a 27.8% infusion ratio was determined using the maximum AUC point of the ROC curve (Figure 1). In regards to optimal infusion volume, Jin et al.12), recommended an infusion ratio of 11.64% which presented sensitivity and specificity of 37% and 93%, whereas 24% by Nieuwenhuijse et al.24), showed sensitivity and specificity of 80.0% and 64%. Comparing our report with these two reports, our results showed higher reliability, since both sensitivity and specificity was high as 80.0% and 87.5%, respectively.
This study has limitations in that since many patients have short follow-up period, our data does not yet provide definitive conclusions on the efficacy of the procedure. Another weakness of this study was that VAS score was solely utilized as an indicator of clinical outcome, so that the study had limits in reflecting functional outcome. The correlation between the types and degrees of fracture and the clinical outcome was yet analyzed. Multivariate analysis including more variables is required.
Go to : 
This study showed high cement ratio revealed favorable outcome in the vertebroplasty of the osteoporotic compression fractures. Infusion ratio of more than 27.8% to osteoporotic compressed vertebrae is optimal. In order to obtain better clinical outcome, calculation of the target volume of cement using infusion ratio before vertebroplasty is recommended.
Go to : 
References
1. Al-Ali F, Barrow T, Luke K. Vertebroplasty: what is important and what is not. AJNR Am J Neuroradiol. 2009; 30:1835–1839. PMID: 19713320.

2. Baumann C, Fuchs H, Kiwit J, Westphalen K, Hierholzer J. Complications in percutaneous vertebroplasty associated with puncture or cement leakage. Cardiovasc Intervent Radiol. 2007; 30:161–168. PMID: 17216377.

3. Belkoff SM, Mathis JM, Jasper LE, Deramond H. The biomechanics of vertebroplasty. The effect of cement volume on mechanical behavior. Spine (Phila Pa 1976). 2001; 26:1537–1541. PMID: 11462082.
4. Boszczyk B. Volume matters: a review of procedural details of two randomised controlled vertebroplasty trials of 2009. Eur Spine J. 2010; 19:1837–1840. PMID: 20686793.

5. Dahl OE, Garvik LJ, Lyberg T. Toxic effects of methylmethacrylate monomer on leukocytes and endothelial cells in vitro. Acta Orthop Scand. 1994; 65:147–153. PMID: 8197846.

6. Deramond H, Wright NT, Belkoff SM. Temperature elevation caused by bone cement polymerization during vertebroplasty. Bone. 1999; 25:17S–21S. PMID: 10458268.

7. Galibert P, Deramond H, Rosat P, Le Gars D. Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty. Neurochirurgie. 1987; 33:166–168. PMID: 3600949.
8. Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993; 8:1137–1148. PMID: 8237484.

9. Graham J, Ahn C, Hai N, Buch BD. Effect of bone density on vertebral strength and stiffness after percutaneous vertebroplasty. Spine (Phila Pa 1976). 2007; 32:E505–E511. PMID: 17700430.

10. Harrop JS, Prpa B, Reinhardt MK, Lieberman I. Primary and secondary osteoporosis’ incidence of subsequent vertebral compression fractures after kyphoplasty. Spine (Phila Pa 1976). 2004; 29:2120–2125. PMID: 15454702.

11. Jensen ME, Evans AJ, Mathis JM, Kallmes DF, Cloft HJ, Dion JE. Percutaneous polymethylmethacrylate vertebroplasty in the treatment of osteoporotic vertebral body compression fractures: technical aspects. AJNR Am J Neuroradiol. 1997; 18:1897–1904. PMID: 9403451.
12. Jin YJ, Yoon SH, Park KW, Chung SK, Kim KJ, Yeom JS, et al. relationship with clinical outcome and complications. Spine (Phila Pa 1976). 2011; 36:E761–E772. PMID: 21289575.
13. Kaufmann TJ, Trout AT, Kallmes DF. The effects of cement volume on clinical outcomes of percutaneous vertebroplasty. AJNR Am J Neuroradiol. 2006; 27:1933–1937. PMID: 17032870.
14. Kim JM, Shin DA, Byun DH, Kim HS, Kim S, Kim HI. Effect of bone cement volume and stiffness on occurrences of adjacent vertebral fractures after vertebroplasty. J Korean Neurosurg Soc. 2012; 52:435–440. PMID: 23323162.

15. Leeson MC, Lippitt SB. Thermal aspects of the use of polymethylmethacrylate in large metaphyseal defects in bone. A clinical review and laboratory study. Clin Orthop Relat Res. 1993; (295):239–245.
16. Liebschner MA, Rosenberg WS, Keaveny TM. Effects of bone cement volume and distribution on vertebral stiffness after vertebroplasty. Spine (Phila Pa 1976). 2001; 26:1547–1554. PMID: 11462084.

17. Luo J, Daines L, Charalambous A, Adams MA, Annesley-Williams DJ, Dolan P. Vertebroplasty: only small cement volumes are required to normalize stress distributions on the vertebral bodies. Spine (Phila Pa 1976). 2009; 34:2865–2873. PMID: 20010394.
18. Luo J, Skrzypiec DM, Pollintine P, Adams MA, Annesley-Williams DJ, Dolan P. Mechanical efficacy of vertebroplasty: influence of cement type, BMD, fracture severity, and disc degeneration. Bone. 2007; 40:1110–1119. PMID: 17229596.

19. Lyles KW, Gold DT, Shipp KM, Pieper CF, Martinez S, Mulhausen PL. Association of osteoporotic vertebral compression fractures with impaired functional status. Am J Med. 1993; 94:595–601. PMID: 8506884.

20. Martinčič D, Brojan M, Kosel F, Štern D, Vrtovec T, Antolič V, et al. Minimum cement volume for vertebroplasty. Int Orthop. 2015; 39:727–733. PMID: 25500712.

21. Mjöberg B, Pettersson H, Rosenqvist R, Rydholm A. Bone cement, thermal injury and the radiolucent zone. Acta Orthop Scand. 1984; 55:597–600. PMID: 6524324.

22. Molloy S, Riley LH 3rd, Belkoff SM. Effect of cement volume and placement on mechanical-property restoration resulting from vertebroplasty. AJNR Am J Neuroradiol. 2005; 26:401–404. PMID: 15709144.
23. Nam DH, Park KH, Kim TW, Chi MP, Kim JO. The effect of trauma in osteoporotic vertebral compression fractures treated by percutaneous vertebroplasty: a comparison of radiological features in presence or absence of trauma. J Korean Neurotraumatol Soc. 2011; 7:29–34.

24. Nieuwenhuijse MJ, Bollen L, van Erkel AR, Dijkstra PD. Optimal intravertebral cement volume in percutaneous vertebroplasty for painful osteoporotic vertebral compression fractures. Spine (Phila Pa 1976). 2012; 37:1747–1755. PMID: 22433500.

25. Nieuwenhuijse MJ, van Rijswijk CS, van Erkel AR, Dijkstra SP. The intravertebral cleft in painful long-standing osteoporotic vertebral compression fractures treated with percutaneous vertebroplasty: diagnostic assessment and clinical significance. Spine (Phila Pa 1976). 2012; 37:974–981. PMID: 22020580.
26. Ploeg WT, Veldhuizen AG, The B, Sietsma MS. Percutaneous vertebroplasty as a treatment for osteoporotic vertebral compression fractures: a systematic review. Eur Spine J. 2006; 15:1749–1758. PMID: 16823557.

27. Ryu KS, Park CK, Kim MC, Kang JK. Dose-dependent epidural leakage of polymethylmethacrylate after percutaneous vertebroplasty in patients with osteoporotic vertebral compression fractures. J Neurosurg. 2002; 96:56–61. PMID: 11795714.

28. Yeom JS, Kim WJ, Choy WS, Lee CK, Chang BS, Kang JW. Leakage of cement in percutaneous transpedicular vertebroplasty for painful osteoporotic compression fractures. J Bone Joint Surg Br. 2003; 85:83–89. PMID: 12585583.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download