1. Aarabi B, Hesdorffer DC, Ahn ES, Aresco C, Scalea TM, Eisenberg HM. Outcome following decompressive craniectomy for malignant swelling due to severe head injury. J Neurosurg. 2006; 104:469–467. PMID:
16619648.

2. Akins PT, Guppy KH. Sinking skin flaps, paradoxical herniation, and external brain tamponade: a review of decompressive craniectomy management. Neurocrit Care. 2008; 9:269–276. PMID:
18064408.

3. Albanèse J, Leone M, Alliez JR, Kaya JM, Antonini F, Alliez B, et al. Decompressive craniectomy for severe traumatic brain injury: evaluation of the effects at one year. Crit Care Med. 2003; 31:2535–2538. PMID:
14530763.

4. Brain Trauma Foundation. American Association of Neurological Surgeons. Congress of Neurological Surgeons. Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007; 24(Suppl 1):S1–S106.
5. Bullock MR, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW, et al. Surgical management of acute subdural hematomas. Neurosurgery. 2006; 58(3 Suppl):S16–S24. discussion Si-SivPMID:
16710968.

6. Cavuşoğ lu H, Kaya RA, Türkmenoğlu ON, Aydin Y. Value of early unilateral decompressive craniectomy in patients with severe traumatic brain injury. Ulus Travma Acil Cerrahi Derg. 2010; 16:119–124. PMID:
20517764.
7. Cianchi G, Bonizzoli M, Zagli G, di Valvasone S, Biondi S, Ciapetti M, et al. Late decompressive craniectomyafter traumatic brain injury: neurological outcome at 6 months after ICU discharge. J Trauma Manag Outcomes. 2012; 6:8. PMID:
22867014.

8. Cooper DJ, Rosenfeld JV, Murray L, Arabi YM, Davies AR, D'Urso P, et al. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med. 2011; 364:1493–1502. PMID:
21434843.

9. Cooper DJ, Rosenfeld JV, Murray L, Wolfe R, Ponsford J, Davies A, et al. Early decompressive craniectomy for patients with severe traumatic brain injury and refractory intracranial hypertension--a pilot randomized trial. J Crit Care. 2008; 23:387–393. PMID:
18725045.

10. Csókay A, Emelifeonwu JA, Fügedi L, Valálik I, Láng J. The importance of very early decompressive craniectomy as a prevention to avoid the sudden increase of intracranial pressure in children with severe traumatic brain swelling (retrospective case series). Childs Nerv Syst. 2012; 28:441–444. PMID:
22207401.

11. Eberle BM, Schnüriger B, Inaba K, Gruen JP, Demetriades D, Belzberg H. Decompressive craniectomy: surgical control of traumatic intracranial hypertension may improve outcome. Injury. 2010; 41:894–898. PMID:
21574279.

12. Figaji AA, Fieggen AG, Peter JC. Early decompressive craniotomy in children with severe traumatic brain injury. Childs Nerv Syst. 2003; 19:666–673. PMID:
12908115.

13. Gaab MR, Rittierodt M, Lorenz M, Heissler HE. Traumatic brain swelling and operative decompression: a prospective investigation. Acta Neurochir Suppl (Wien). 1990; 51:326–328. PMID:
2089928.

14. Guerra WK, Gaab MR, Dietz H, Mueller JU, Piek J, Fritsch MJ. Surgical decompression for traumatic brain swelling: indications and results. J Neurosurg. 1999; 90:187–196. PMID:
9950487.

15. Helmy A, Vizcaychipi M, Gupta AK. Traumatic brain injury: intensive care management. Br J Anaesth. 2007; 99:32–42. PMID:
17556349.

16. Hutchinson PJ, Menon DK, Kirkpatrick PJ. Decompressive craniectomy in traumatic brain injury--time for randomised trials? Acta Neurochir (Wien). 2005; 147:1–3. PMID:
15614466.
17. Jiang JY, Xu W, Li WP, Xu WH, Zhang J, Bao YH, et al. Efficacy of standard trauma craniectomy for refractory intracranial hypertension with severe traumatic brain injury: a multicenter, prospective, randomized controlled study. J Neurotrauma. 2005; 22:623–628. PMID:
15941372.

18. Juul N, Morris GF, Marshall SB, Marshall LF. Intracranial hypertension and cerebral perfusion pressure: influence on neurological deterioration and outcome in severe head injury. The Executive Committee of the International Selfotel Trial. J Neurosurg. 2000; 92:1–6. PMID:
10616075.
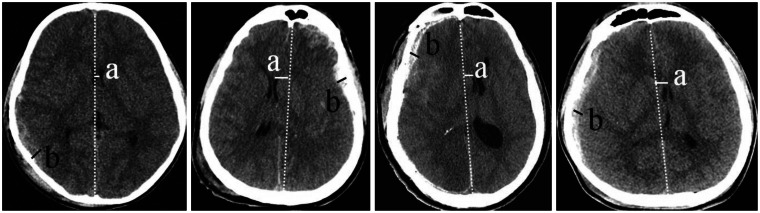
19. Kim JY, Hyun DK, Yoon SH, Park HS, Kim EY, Park HC, et al. Prediction of postoperative drainage volume and brain expansion of chronic subdural hematoma: supplementary study-clinical study. J Korean Neurotraumatol Soc. 2010; 6:33–37.

20. Kunze E, Meixensberger J, Janka M, Sörensen N, Roosen K. Decompressive craniectomy in patients with uncontrollable intracranial hypertension. Acta Neurochir Suppl. 1998; 71:16–18. PMID:
9779131.

21. Münch E, Horn P, Schürer L, Piepgras A, Paul T, Schmiedek P. Management of severe traumatic brain injury by decompressive craniectomy. Neurosurgery. 2000; 47:315–322. discussion 322-323PMID:
10942004.

22. Marshall LF. Head injury: recent past, present, and future. Neurosurgery. 2000; 47:546–561. PMID:
10981741.

23. Marshall LF, Smith RW, Shapiro HM. The outcome with aggressive treatment in severe head injuries. Part I: the significance of intracranial pressure monitoring. J Neurosurg. 1979; 50:20–25. PMID:
758374.
24. Meier U, Ahmadi S, Killeen T, Al-Zain FT, Lemcke J. Long-term outcomes following decompressive craniectomy for severe head injury. Acta Neurochir Suppl. 2008; 102:29–31. PMID:
19388283.
25. Menon DK. Cerebral protection in severe brain injury: physiological determinants of outcome and their optimisation. Br Med Bull. 1999; 55:226–258. PMID:
10695089.

26. Messing-Jünger AM, Marzog J, Wöbker G, Sabel M, Bock WJ. Decompressive craniectomy in severe brain injury. Zentralbl Neurochir. 2003; 64:171–177. PMID:
14634882.

27. Miller JD, Becker DP, Ward JD, Sullivan HG, Adams WE, Rosner MJ. Significance of intracranial hypertension in severe head injury. J Neurosurg. 1977; 47:503–516. PMID:
903804.

28. Oh CH, Hyun DK, Yoon SH, Park HS, Kim EY, Park HC, et al. The relationship between postoperative drainage volume and brain shifting index in chronic subdural hematoma. J Korean Neurotraumatol Soc. 2008; 4:70–76.

29. Oh CH, Park CO, Hyun DK, Park HC, Yoon SH. Comparative study of outcomes between shunting after cranioplasty and in cranioplasty after shunting in large concave flaccid cranial defect with hydrocephalus. J Korean Neurosurg Soc. 2008; 44:211–216. PMID:
19096679.

30. Piek J. Decompressive surgery in the treatment of traumatic brain injury. Curr Opin Crit Care. 2002; 8:134–138. PMID:
12386514.

31. Polin RS, Shaffrey ME, Bogaev CA, Tisdale N, Germanson T, Bocchicchio B, et al. Decompressive bifrontal craniectomy in the treatment of severe refractory posttraumatic cerebral edema. Neurosurgery. 1997; 41:84–92. discussion 92-94PMID:
9218299.

32. Sahuquillo J, Arikan F. Decompressive craniectomy for the treatment of refractory high intracranial pressure in traumatic brain injury. Cochrane Database Syst Rev. 2006; CD003983. PMID:
16437469.

33. Schirmer CM, Ackil AA Jr, Malek AM. Decompressive Craniectomy. Neurocrit Care. 2008; 8:456–470. PMID:
18392785.

34. Stiver SI. Complications of decompressive craniectomy for traumatic brain injury. Neurosurg Focus. 2009; 26:E7. PMID:
19485720.

35. Taylor A, Butt W, Rosenfeld J, Shann F, Ditchfield M, Lewis E, et al. A randomized trial of very early decompressive craniectomy in children with traumatic brain injury and sustained intracranial hypertension. Childs Nerv Syst. 2001; 17:154–162. PMID:
11305769.

36. Timofeev I, Czosnyka M, Nortje J, Smielewski P, Kirkpatrick P, Gupta A, et al. Effect of decompressive craniectomy on intracranial pressure and cerebrospinal compensation following traumatic brain injury. J Neurosurg. 2008; 108:66–73. PMID:
18173312.

37. Ucar T, Akyuz M, Kazan S, Tuncer R. Role of decompressive surgery in the management of severe head injuries: prognostic factors and patient selection. J Neurotrauma. 2005; 22:1311–1318. PMID:
16305319.

38. Wen L, Wang H, Wang F, Gong JB, Li G, Huang X, et al. A prospective study of early versus late craniectomy after traumatic brain injury. Brain Inj. 2011; 25:1318–1324. PMID:
21902550.

39. Williams RF, Magnotti LJ, Croce MA, Hargraves BB, Fischer PE, Schroeppel TJ, et al. Impact of decompressive craniectomy on functional outcome after severe traumatic brain injury. J Trauma. 2009; 66:1570–1574. discussion 1574-1576PMID:
19509616.

40. Woertgen C, Rothoerl RD, Schebesch KM, Albert R. Comparison of craniotomy and craniectomy in patients with acute subdural haematoma. J Clin Neurosci. 2006; 13:718–721. PMID:
16904897.

41. Xiao B, Wu FF, Zhang H, Ma YB. A randomized study of urgent computed tomography-based hematoma puncture and aspiration in the emergency department and subsequent evacuation using craniectomy versus craniectomy only. J Neurosurg. 2012; 117:566–573. PMID:
22769066.

42. Yang XF, Wen L, Shen F, Li G, Lou R, Liu WG, et al. Surgical complications secondary to decompressive craniectomy in patients with a head injury: a series of 108 consecutive cases. Acta Neurochir (Wien). 2008; 150:1241–1247. discussion 1248PMID:
19005615.

43. Yang XJ, Hong GL, Su SB, Yang SY. Complications induced by decompressive craniectomies after traumatic brain injury. Chin J Traumatol. 2003; 6:99–103. PMID:
12659705.
44. Yoo DS, Kim DS, Cho KS, Huh PW, Park CK, Kang JK. Ventricular pressure monitoring during bilateral decompression with dural expansion. J Neurosurg. 1999; 91:953–959. PMID:
10584840.

45. Ziai WC, Port JD, Cowan JA, Garonzik IM, Bhardwaj A, Rigamonti D. Decompressive craniectomy for intractable cerebral edema: experience of a single center. J Neurosurg Anesthesiol. 2003; 15:25–32. PMID:
12499979.

46. Zweckberger K, Stoffel M, Baethmann A, Plesnila N. Effect of decompression craniotomy on increase of contusion volume and functional outcome after controlled cortical impact in mice. J Neurotrauma. 2003; 20:1307–1314. PMID:
14748979.





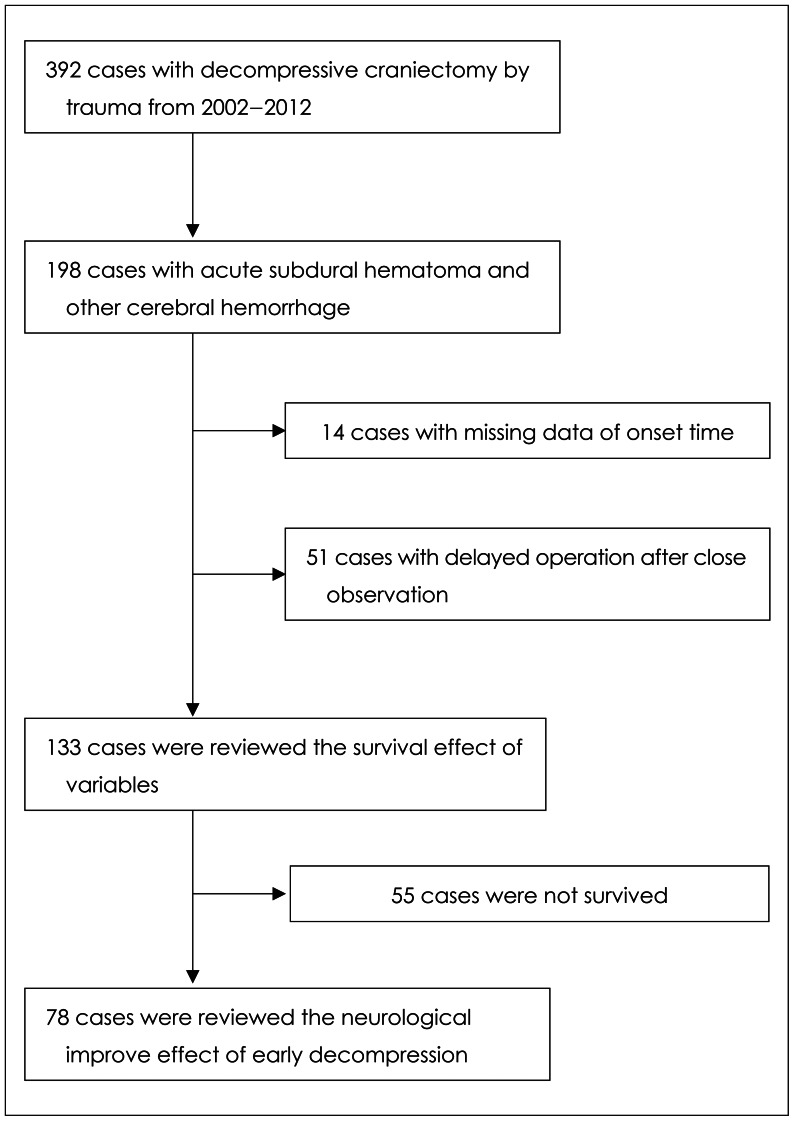
 PDF
PDF ePub
ePub Citation
Citation Print
Print



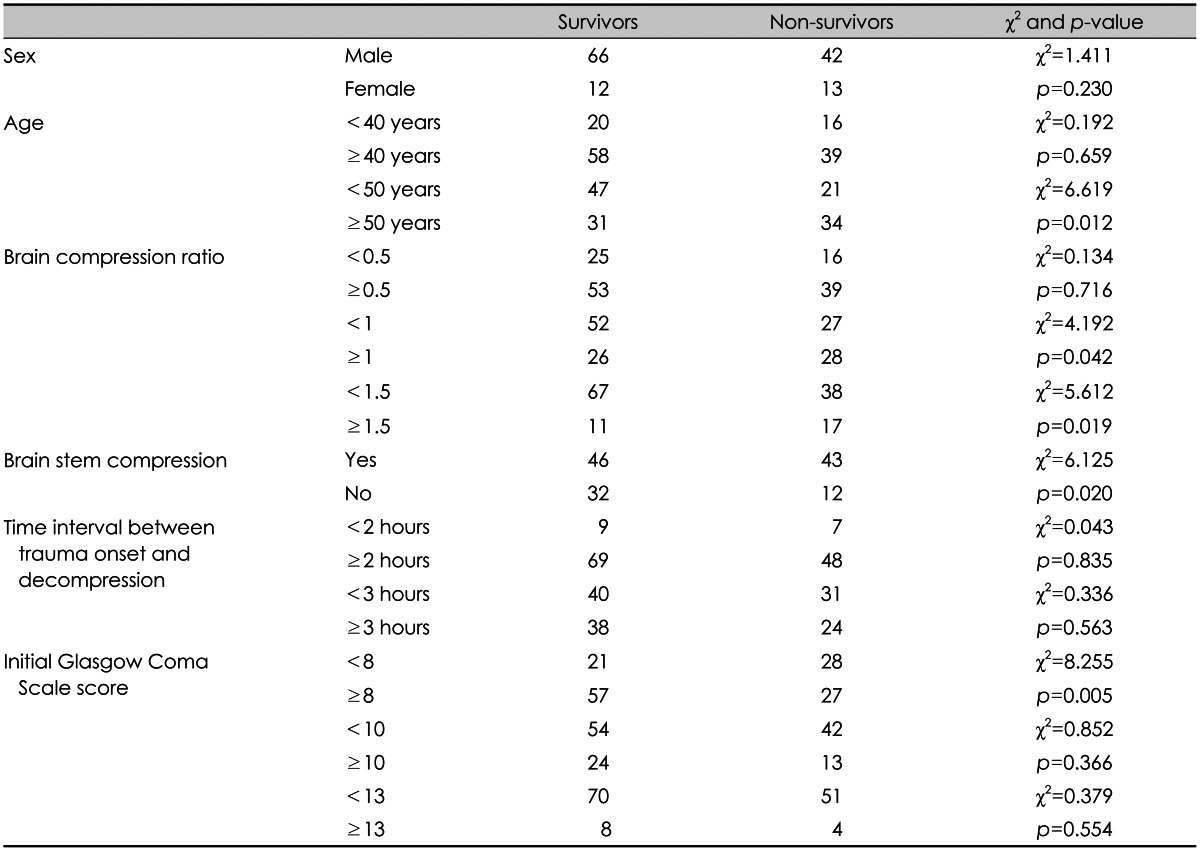
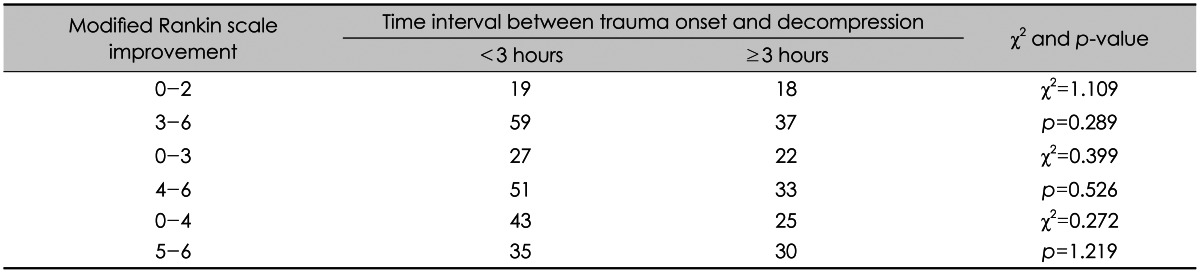
 XML Download
XML Download