Abstract
Objective
Although twist-drill craniostomy (TDC) has a number of procedural advantages and an equivalent outcome compared to burr hole craniostomy (BHC) for the treatment of chronic subdural hematomas (CSDHs), the latter technique remains the preferred method. We analyzed symptomatic CSDHs in whom TDC at the pre-coronal suture entry point (PCSEP) was the primary method for hematoma drainage and BHC on the parietal was the secondary option.
Methods
CSDHs in 86 consecutive patients were included. TDC at the PCSEP, which is 1 cm anterior to coronal suture at the level of the superior temporal line, was the primary operational technique when the hematoma thickness was suitable, and BHC was performed via the parietal when TDC was unreasonable or failed. The clinical feasibility and outcomes of these approaches were analyzed.
Results
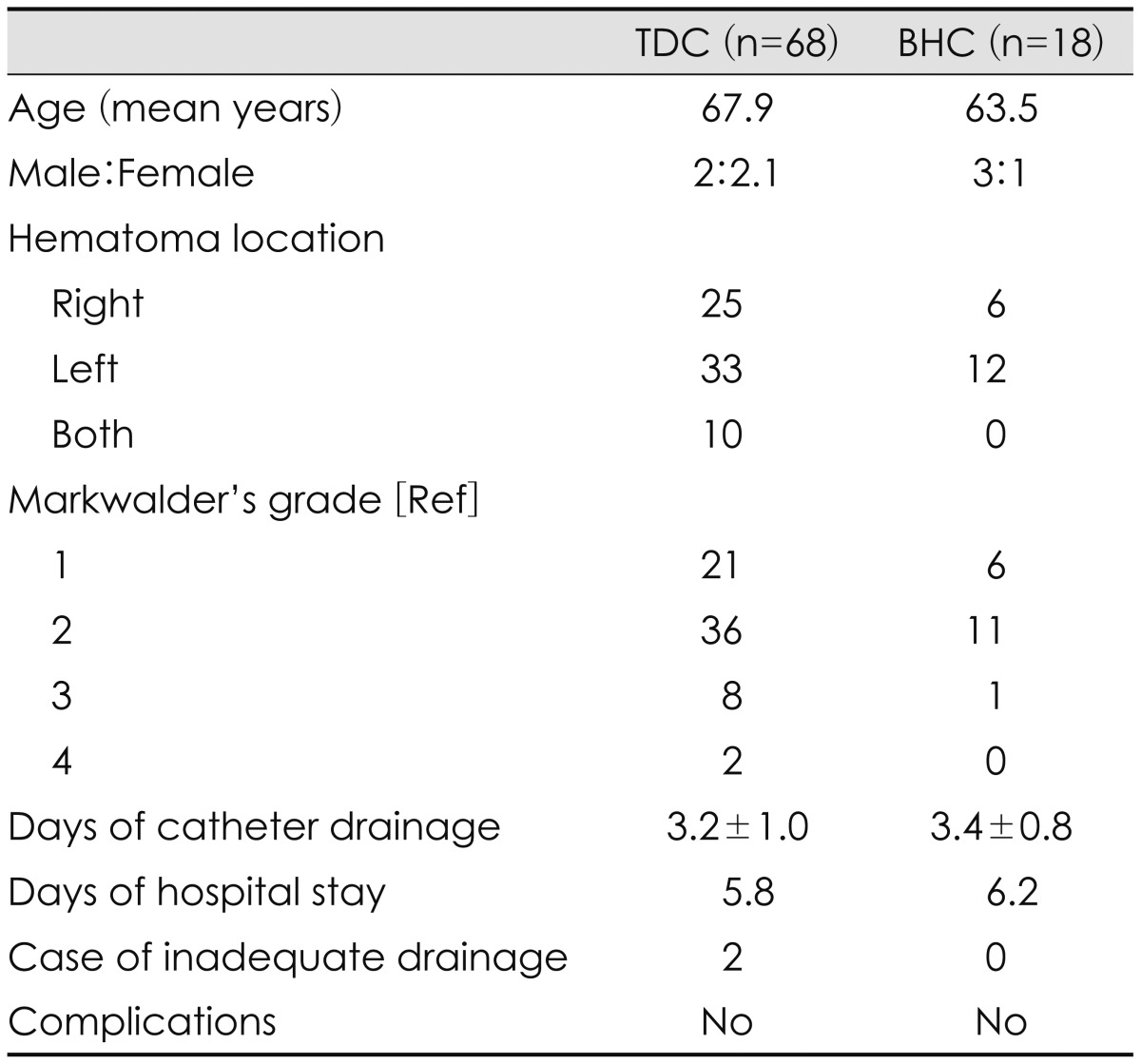
Of the 86 patients, 68 (79.1%) were treated by TDC, and 18 (20.9%) by BHC. All patients showed improvements in their symptoms after hematoma drainage. Neither morbidity nor mortality was associated with either technique, and there were no differences in drainage days between the groups. Ten patients had bilateral hematomas and were treated using TDC. Two patients were not sufficiently treated by TDC and, as a result, BHC was applied. Only six hematomas (7% of 86 hematomas) exhibited insufficient thickness on the computed tomography to perform TDC.
Go to : 
Although chronic subdural hematomas (CSDHs) are a frequently encountered neurological condition, there has yet to be a firm consensus regarding the optimal surgical technique for the treatment of this disorder.1121820) However, based on the disease-prevalent population, surgical morbidities, and recurrence after surgery, less-invasive surgical techniques have become the initial procedures of choice.912171923) Closed-system drainage using burr hole craniostomy (BHC) or twist-drill craniostomy (TDC) have been recommended as first-choice treatments for CSDHs, but the method chosen is based on the surgeon's preference.
We have proposed that a safe entry point for the treatment of CSDHs using TDC is 1 cm anterior to the coronal suture at the superior temporal line.6) This entry point, which is known as the pre-coronal suture entry point (PCSEP), allows for safe trephination due to prominent skull landmarks as well as the preoperative estimation of hematoma thickness using a brain computed tomography (CT) scan. The safety and technical usefulness of TDC at PCSEP have been confirmed in the literature.813) Thus, the aim of the present study was to determine the surgical experiences and patient outcomes of prospectively collected data for consecutive patients who were treated by the surgeon who originally developed the PCSEP approach.
Go to : 
The present study enrolled patients with surgically-treated CSDHs who were managed by a single surgeon (senior author: SCH) and whose data were prospectively collected over 5 consecutive years. The symptomatic CSDHs of 86 patients who had follow-up periods of more than 3 months were included in this study. When the hematoma had a thickness of more than twice that of the skull at the normal entry point, the first-choice technique was TDC via the PCSEP, which was 1 cm anterior to the coronal suture in the superior temporal line. This point is easily indicated on a brain CT scan and on the patient's scalp. If TDC was not possible, BHC was performed at the parietal entry point because this area would be more aesthetically acceptable for a longer scalp incision and the postoperative depression of the skull. Additionally, on trying TDC at the parietal area, we did not intend to make an injury to the meningeal vessels and penetrate the brain when the exact thickness of the hematoma was uncertain. The choice of procedural methods for treating the CSDHs was simplified by using TDC at PCSEP as the primary treatment option and BHC at the parietal bossing area as a secondary treatment option. The clinical characteristics of both groups were assessed to determine how many TDC procedures were performed in consecutive CSDH patients and the reasons that BHC would be performed as secondary option.
The TDC surgical procedure used in the present study was identical to that previously described by our research group.6) The PCSEP was marked on the scalp, hematoma thickness at the PCSEP was easily determined using a brain CT scan, and TDC was performed if the thickness of the hematoma was more than twice the thickness of the bone. After scalp preparation with alcohol and a povidone-iodine solution, a solution of 2% lidocaine was applied, and a stab incision approximately 5 mm in length was made with a No. 15 scalpel at the entry point. First, the skull and dura were penetrated in a perpendicular manner with a hand drill to avoid the twist-drill's slipping down the skull and separating the dura from the skull. Then, the twist-drill trephination was made at a 45° angle to the surface of the bone to prevent the indwelling catheter from entering the cortex; the usual direction is posteroinferior toward the auricle. A standard ventriculostomy catheter (No. 5) was introduced into the subdural cavity at a depth of approximately 5 cm, and the contents were allowed to flow freely to and fro according to the up and down movement of the catheter. Neither aspiration with negative pressure nor flushing with normal saline was performed.
For the BHC procedure, a vertical scalp incision was made on the parietal bossing area, and a burr hole was created with an air drill. Following the dural incision, the ventriculostomy catheter was inserted into the hematoma cavity in an anteroinferior direction. No irrigation was done through the burr hole.
Go to : 
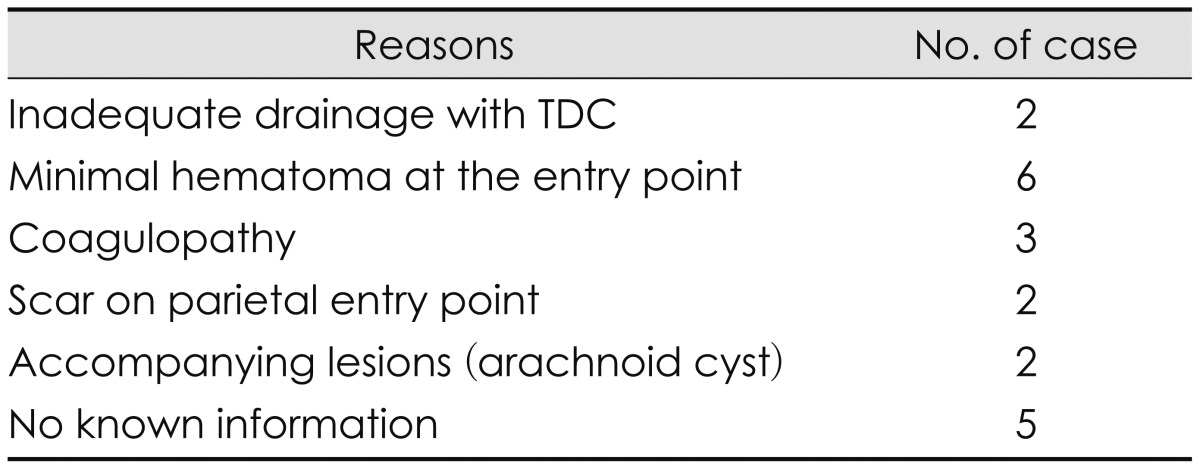
The present study included 86 patients; 68 (79.1%) were treated by TDC, and 18 (20.9%) by BHC as the initial treatment method (Table 1). All patients showed improvements in their symptoms after hematoma drainage. Furthermore, no morbidity or mortality was associated with either technique, and there were no differences in duration of drainage days or hospital stay between the groups (Table 1). Remarkably, no BHC procedures were necessary for patients with bilateral CSDHs because each of these cases had enough thickness at the PCSEP to accommodate the trephination and insertion of a drainage catheter. The reasons for the performance of BHC in 20 patients are shown in Table 2; in two cases, the hematomas were insufficiently drained by the initial TDC procedure and, subsequently, BHC was performed at the parietal entry point 1 day later. Six patients had hematomas (7% of 86 hematomas) that did not exhibit sufficient thickness on the CT scan to warrant a TDC procedure. Three had the coagulopathy as an underlying disease, so burr hole drainage was done as the first treatment. Two had a previous scar on the parietal entry point, two had associated lesions (such as an arachnoid cyst), and a retrospective analysis could not identify the reason for a BHC as the first-choice option in five patients. So, twelve hematomas of those patients could be drained by TDC instead of BHC as a first treatment method.
Go to : 
The drainage of CSDHs can be accomplished by two craniostomy methods: the burr hole and twist-drill techniques. Because there are no significant differences in the effectiveness of these two techniques,112) the surgeon's preference determines their use. Thus, the present study aimed to determine the safety and feasibility of TDC at the PCSEP as a first-choice treatment option for CSDHs using 86 prospectively enrolled patients. TDC was attempted in 79.1% of the patients in the present study. However, approximately 93.0% (80 of 86) of the patients may have been treated with the TDC technique if the BHC cases had been treated with TDC, except for six patients with minimal hematomas at the PCSEP. Only 7% of the hematomas exhibit not enough thickness at the PCSEP on a brain CT scan to consider the use of TDC. In our case series for draining CSDHs, only 2 cases had insufficient drainage by initial TDC procedure. Nonetheless, the BHC cases decreased as the opportunities to perform TDC increased.
Because a craniostomy is a relatively less invasive procedure, it is the favored treatment method for symptomatic CSDHs. Surgery and anesthesia entail the invasive techniques and are associated with medical complications; thus, the least invasive operative therapies for CSDHs hypothetically offer the best chance to reduce mortality after surgery.182124) The use of a postoperative continuous closed-system drainage following TDC or BHC offers substantial advantages for the treatment of CSDHs by allowing the brain to sufficiently re-expand and fill the subdural space.22) Additionally, the closed catheter drainage system reduces the likelihood of a recurrence of the hematoma.1912) The incomplete removal or reaccumulation of a hematoma frequently occurs after BHC,1821) but the maturation of the neomembrane can be a primary mechanism underlying the spontaneous resolution of a remnant hematoma that can prevent hematoma reaccumulation.9) Operations that are relatively more invasive, such as larger craniotomies, are not more effective in terms of hematoma evacuation, the avoidance of hematoma reaccumulation, or neurological improvement.4) In fact, rebleeding from the hematoma neomembrane makes the incomplete removal and/or reaccumulation of a hematoma effectively inevitable regardless of the surgical method.18) Taken together, these findings suggest that the simple continuous drainage of CSDHs is the treatment of choice, even though there are no significant differences in the recurrence rates after BHC and TDC.1)
TDC is an effective treatment modality for CSDHs,192122) and if the TDC procedure ensures the safety of the patient during the operation, then it can be a first-choice treatment option for CSDHs. Before the PCSEP was introduced as a normal entry point, craniostomy procedures were performed in indefinite areas, such as the rostral or anterior aspects,2523) or the site of maximal thickness of the subdural hematoma.161925) The indefinite nature of these procedures resulted in what would be an essentially blind TDC operation in which the site of trephination may be in a different location than that determined by the preoperative design and may be in an area along the dural vasculature. As a result, surgeons neglected the advantages of the TDC operation, such as a shorter procedure time, less scarring, and the avoidance of air entrapment.16) Air entrapment is a risk factor for recurrence.15)
The PCSEP, which is 1 cm anterior to the coronal suture at the level of superior temporal line, has many advantages including anatomical safety and a navigating landmark. First, because it is located at a relatively frontal position, the procedure can be performed in the supine position, and the patient feels comfortable and can be easily monitored if sedated. Second, both sides of the PCSEP can be trephined without a surgical position change in the case of bilateral hematoma; in the present study, all of the bilateral hematomas were treated by TDC. Third, the PCSEP is close to the coronal suture, which enhances the adhesion of the dura to the skull and results in decreased risks of dural detachment and postoperative epidural hematoma. Finally, the shorter procedure time for TDC, with a mean operation duration of only 8.9 minutes,13) may obviate the need for monitored or general anesthesia, especially in confused or elderly patients. Additionally, even though a patient may be bald in the frontal region, TDC may not be contraindicated because it involves a stab incision and not a bony depression.
Although CSDHs are generally considered benign,31114) many elderly patients suffer from this disorder and exhibit a high mortality rate for up to 1 year after their diagnosis relative to the anticipated actuarial survival.14) This suggests that CSDHs are a marker of other underlying chronic diseases, such as hip fracture. However, we found that many patients included in the study do not undergo surgery (34.4%), some have a relatively high in-hospital mortality rate (16.7%), and few return home (21.1%).14) Nonetheless, the surgical drainage of CSDHs in nonagenarians and centenarians is associated with a lower incidence of inpatient death and higher 30-day and 6-month survival rates.10) And none of the patients in the conservative care group showed any neurological improvement10); thus, the aggressive surgical drainage of symptomatic CSDHs may enhance the survival of elderly patients.
In the elderly, coexisting systemic diseases typically pose a problem under general anesthesia. Although there are no significant differences in terms of surgical complications between patients who receive local and general anesthesia, those who undergo general anesthesia exhibit more cardiovascular complications and longer hospitalization periods.7) Thus, the shorter procedural duration of TDC and the absence of sedation or general anesthesia during the procedure may help reduce the morbidity. The draining of a hematoma should be not accompanied by morbidity that could result in a high mortality rate, and the least invasive and shortest procedures should be considered as first-choice treatment options, while more invasive surgical techniques should be reserved for more complicated situations. Thus, it is proposed here that TDC at the PCSEP should be considered a first-choice treatment option for patients with CSDH, especially if they are elderly.
Go to : 
In conclusion, the majority of the CSDHs in the present study were effectively treated by TDC at the PCSEP and a closed drainage system when the hematoma was of sufficient thickness. Thus, the present findings suggest that TDC at the PCSEP should be considered a first-choice treatment option for CSDHs, especially in elderly patients, while BHC at the parietal entry point should be reserved for more complicated cases.
Go to : 
References
1. Almenawer SA, Farrokhyar F, Hong C, Alhazzani W, Manoranjan B, Yarascavitch B, et al. Chronic subdural hematoma management: a systematic review and meta-analysis of 34,829 patients. Ann Surg. 2014; 259:449–457. PMID: 24096761.
2. Camel M, Grubb RL Jr. Treatment of chronic subdural hematoma by twist-drill craniotomy with continuous catheter drainage. J Neurosurg. 1986; 65:183–187. PMID: 3723175.
3. Chen JC, Levy ML. Causes, epidemiology, and risk factors of chronic subdural hematoma. Neurosurg Clin N Am. 2000; 11:399–406. PMID: 10918008.

4. Hamilton MG, Frizzell JB, Tranmer BI. Chronic subdural hematoma: the role for craniotomy reevaluated. Neurosurgery. 1993; 33:67–72. PMID: 8355849.
5. Horn EM, Feiz-Erfan I, Bristol RE, Spetzler RF, Harrington TR. Bedside twist drill craniostomy for chronic subdural hematoma: a comparative study. Surg Neurol. 2006; 65:150–153. discussion 153-154. PMID: 16427409.

6. Hwang SC, Im SB, Kim BT, Shin WH. Safe entry point for twist-drill craniostomy of a chronic subdural hematoma. J Neurosurg. 2009; 110:1265–1270. PMID: 19099378.

7. Kim SO, Jung SI, Won YS, Choi CS, Yang JY. A comparative study of local versus general anesthesia for chronic subdural hematoma in elderly patients over 60 years. Korean J Neurotrauma. 2013; 9:47–51.

8. Lee JY, Kim BT, Hwang SC, Im SB, Shin DS, Shin WH. Indications and surgical results of twist-drill craniostomy at the pre-coronal point for symptomatic chronic subdural hematoma patients. J Korean Neurosurg Soc. 2012; 52:133–137. PMID: 23091672.

9. Lee KS. Natural history of chronic subdural haematoma. Brain Inj. 2004; 18:351–358. PMID: 14742149.
10. Lee L, Ker J, Ng HY, Munusamy T, King NK, Kumar D, et al. Outcomes of chronic subdural hematoma drainage in nonagenarians and centenarians: a multicenter study. J Neurosurg. 2016; 124:546–551. PMID: 26162032.

11. Lega BC, Danish SF, Malhotra NR, Sonnad SS, Stein SC. Choosing the best operation for chronic subdural hematoma: a decision analysis. J Neurosurg. 2010; 113:615–621. PMID: 19877806.

12. Liu W, Bakker NA, Groen RJ. Chronic subdural hematoma: a systematic review and meta-analysis of surgical procedures. J Neurosurg. 2014; 121:665–673. PMID: 24995782.

13. Lu J, Shen D, Hu F, Zhou J, Lan F, Guo D, et al. An improved electronic twist-drill craniostomy procedure with post-operative urokinase instillation in treating chronic subdural hematoma. Clin Neurol Neurosurg. 2015; 136:61–65. PMID: 26067723.

14. Miranda LB, Braxton E, Hobbs J, Quigley MR. An improved electronic twist-drill craniostomy procedure with post-operative urokinase instillation in treating chronic subdural hematoma. J Neurosurg. 2011; 114:72–76. PMID: 20868215.
15. Nakaguchi H, Tanishima T, Yoshimasu N. Factors in the natural history of chronic subdural hematomas that influence their postoperative recurrence. J Neurosurg. 2001; 95:256–262. PMID: 11780895.

16. Reinges MH, Hasselberg I, Rohde V, Kuker W, Gilsbach JM. Prospective analysis of bedside percutaneous subdural tapping for the treatment of chronic subdural haematoma in adults. J Neurol Neurosurg Psychiatry. 2000; 69:40–47. PMID: 10864602.

17. Richter HP, Klein HJ, Schäfer M. Chronic subdural haematomas treated by enlarged burr-hole craniotomy and closed system drainage. Retrospective study of 120 patients. Acta Neurochir (Wien). 1984; 71:179–188. PMID: 6741635.

18. Rohde V, Graf G, Hassler W. Complications of burr-hole craniostomy and closed-system drainage for chronic subdural hematomas: a retrospective analysis of 376 patients. Neurosurg Rev. 2002; 25:89–94. PMID: 11954771.

19. Rychlicki F, Recchioni MA, Burchianti M, Marcolini P, Messori A, Papo I. Percutaneous twist-drill craniostomy for the treatment of chronic subdural haematoma. Acta Neurochir (Wien). 1991; 113:38–41. PMID: 1799141.

20. Santarius T, Kirkpatrick PJ, Ganesan D, Chia HL, Jalloh I, Smielewski P, et al. Use of drains versus no drains after burr-hole evacuation of chronic subdural haematoma: a randomised controlled trial. Lancet. 2009; 374:1067–1073. PMID: 19782872.

21. Smely C, Madlinger A, Scheremet R. Chronic subdural haematoma-a comparison of two different treatment modalities. Acta Neurochir (Wien). 1997; 139:818–825. discussion 825-826. PMID: 9351986.
22. Tabaddor K, Shulmon K. Definitive treatment of chronic subdural hematoma by twist-drill craniostomy and closed-system drainage. J Neurosurg. 1977; 46:220–226. PMID: 833639.

23. Voelker JL, Sambasivan M. The role of craniotomy and trephination in the treatment of chronic subdural hematoma. Neurosurg Clin N Am. 2000; 11:535–540. PMID: 10918026.

24. Weigel R, Schmiedek P, Krauss JK. Outcome of contemporary surgery for chronic subdural haematoma: evidence based review. J Neurol Neurosurg Psychiatry. 2003; 74:937–943. PMID: 12810784.

25. Williams GR, Baskaya MK, Menendez J, Polin R, Willis B, Nanda A. Burr-hole versus twist-drill drainage for the evacuation of chronic subdural haematoma: a comparison of clinical results. J Clin Neurosci. 2001; 8:551–554. PMID: 11683603.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download