Abstract
Objective
A subdural drain using urokinase after a burr hole hematoma evacuation was performed for subacute subdural hematoma (SASDH), and its effectiveness and safety in elderly patients were evaluated.
Methods
Between January 2013 and May 2015, subdural drains using urokinase after burr hole hematoma evacuation were performed in 19 elderly patients. The inclusion criteria were as follows: 1) a subdural hematoma occurring between 4 and 20 days after injury; 2) worsening neurological symptoms, from mild to moderate or severe, due to injury during the subacute stage; 3) a mix of solid clots (high-density lighter shadow) and fluid hematoma (low-density darker shadow) on the computed tomography (CT) scan; 4) a score of ≥9 on the Glasgow Coma Scale (GCS) assessed immediately before surgery; and 5) an age of ≥65 years. When the majority of the hematoma was evacuated on the CT, we removed the catheter.
Results
Under local anesthesia, a catheter was inserted into the hematoma through a burr hole. The mean age of the patients was 73.7 years (range, 65-87 years). The mean preoperative GCS score was 11.2 (range, 10-13), and the mean Glasgow Outcome Scale score for all patients was 5 at discharge. No recurrences of hematomas or surgical complications were observed.
A hematoma formed between the dura mater and arachnoid is called a subdural hematoma (SDH), and it is known to occur in 5% to 29% of all head trauma patients.1619) It can even occur in patients with no trauma or minor trauma, such as those who take anticoagulants, have a blood disease, or have received a hydrocephalus shunt.7141519) The hematoma is often formed by the rupture of the artery or vein of the cerebral cortex or the bridging vein between the cerebral cortex and the arteries and veins.4)
A subacute SDH (SASDH) is a hematoma occurring between 4 and 20 days after injury.69) Surgical treatment is required if neurological symptoms progress from mild to moderate or severe due to injury during the subacute stage (e.g., decreased consciousness, motor nerve palsy, headache), while patients receive conservative treatment for mild neurological symptoms due to acute SDH.6) For SASDH, a burr hole or craniotomy should be considered. With burr holes, it is often difficult to remove hematomas completely. Although hematomas can be removed completely with craniotomies, they increase morbidity and mortality because of the blood loss, long operating time, postoperative infection, and general anesthesia associated with the invasive surgery.1018)
Therefore, we propose that a subdural drain using urokinase after a burr hole hematoma evacuation under local anesthesia would be less invasive and more effective for elderly patients with SASDHs. Although stereotactic hematoma aspiration using urokinase is commonly practiced for spontaneous intracerebral hemorrhages,211) no studies have been published on its use for SASDH. The effectiveness and safety of a subdural drain using urokinase after a burr hole hematoma evacuation for these patients and their outcomes are reported.
Between January 2013 and May 2015, 19 elderly patients with SASDH who underwent subdural drains using urokinase after burr hole hematoma evacuation were analyzed.
The inclusion criteria were as follows: 1) a SDH occurring between 4 and 20 days after injury; 2) worsening symptoms, from mild to moderate, due to injury during the subacute stage while receiving conservative treatment for mild symptoms due to acute SDH upon admission; 3) a mix of solid clots (high-density lighter shadow) and fluid hematoma (low-density darker shadow) on the computed tomography (CT) scan; 4) a score of ≥9 on the Glasgow Coma Scale (GCS) assessed immediately before surgery; and 5) an age of ≥65 years. The exclusion criteria were as follows: 1) co-occurrence with other head trauma, including brain contusion, epidural hematoma, intracerebral hemorrhage, intraventricular hemorrhage, and traumatic subarachnoid hemorrhage; 2) the occurrence of the SDH as a complication from previous neurosurgery, including craniotomy, ventricular drainage, or ventricle peritoneal shunt; 3) a score of <8 on the GCS assessed immediately before surgery; and 4) an age of <65 years.
Under local anesthesia, a catheter was inserted into the hematoma through a burr hole in all patients. Surgery was conducted with the patient in the supine position with the head raised by approximately 15 degrees and the site for the burr hole raised the highest. The fenestration site was planned for the thickest part of the hematoma. Local anesthesia with 1% lidocaine was applied on the scalp, a 3-cm incision was made, and then fenestration was performed with an electric drill. After incising the dura mater and identifying the membrane of the hematoma, we identified the hematoma from the subdural space and inserted a 9-French drainage tube to a depth of about 5 cm in the frontal area. After injecting a mixture of 3 mL of normal saline and urokinase (1,000 units) using the drainage tube, we closed off the tube for two hours and then opened the tube again to allow the hematoma to be drained naturally by gravity into the drainage pocket. Urokinase was injected into the hematoma every 12 hours. The drainage tube was removed on the second or third day after surgery based on the amount of remaining hematoma seen on the CT performed on the second day after surgery.
We collected the following clinical information on participants: age, gender, symptoms, head trauma history, underlying systemic disease, antiplatelet or anticoagulant medication use, and blood test results. The patient conditions were evaluated using the GCS and assessed immediately before surgery. The treatment outcomes of all patients were evaluated using the Glasgow Outcome Scale (GOS).
This study was approved by the Institutional Review Board (IRB) of the Yeungnam University Hospital (IRB No. YUMC 2015-03-004).
The patient demographic characteristics and clinical features are summarized in Table 1. The mean age of the patients was 73.7 years (range, 65-87 years). There were 13 male and 6 female patients. The causes of injury were slips in 18 patients and a fall from a height in one patient. Underlying diseases included hypertension in five patients, diabetes in eight patients, cerebral infarction in six patients, and myocardial infarction in one patient. Nine patients were taking antiplatelet medication on admission. The blood test results for all patients were normal for prothrombin time, partial thromboplastin time, and platelet counts associated with blood coagulation. The clinical symptoms observed immediately before surgery were drowsy mentality in 10 patients, stupor mentality in two patients, hemiparesis in four patients, gait disturbance in one patient, and sensory aphasia in two patients. Ten of the SASDHs were on the right side, seven were on the left side, and two were bilateral. The mean period in which symptoms worsened after head trauma was 13.6 days (range, 4-20 days). The mean preoperative GCS score was 11.2 (range, 10-13).
The hematoma catheter was in place for a median duration of 2.5 days (range, 2-13 days). The mean GOS score for all patients was 5 at discharge. The mean follow-up period was 17.7 months (range, 12-24 months). During the follow-up period, no recurrences of hematomas or surgery-related complications, such as infection or intracranial hemorrhage, were observed in any of the patients. No bleeding in other organs from the use of urokinase or blood test abnormalities was found.
A 71-year-old female patient was admitted to the emergency room (ER) complaining of headache due to a head trauma after a slip and fall. She showed no neurological abnormalities. The initial CT results showed an acute SDH in the right frontal and temporal regions, resulting in slight pressure on the right ventricle (Figure 1A). She had hypertension, diabetes mellitus, and cerebral infarction in her medical history. She was taking antiplatelet medication on admission. No abnormalities were found on the laboratory analysis. She was initially treated conservatively because of her old age and minor symptoms. On the 17th day from admission, the patient showed symptoms of increased intracranial pressure, including paralysis of the upper and lower-left limbs, an increase in the SDH on the CT, increased pressure on the right ventricle, and aggravation of the midline shift toward the left. The CT showed a mixture of solid blood clots and fluid hematoma in the SDH (Figure 1B). A subdural drain using urokinase after a burr hole hematoma evacuation was performed (Figure 1C). The catheter was removed two days after the surgery based on a CT that showed sufficient removal of the hematoma. The CT at postoperative day 7 showed no recurrence of the hematoma, and the GOS score was 5 at discharge (Figure 1D).
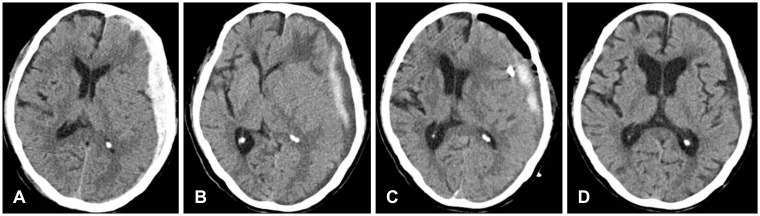
A 79-year-old male patient was admitted to the ER complaining of sensory aphasia and cognitive deterioration due to head trauma after a slip and fall. The initial CT showed an acute SDH in the left frontal and temporal regions, resulting in slight pressure on the left ventricle (Figure 2A). His medical history was unremarkable. No abnormalities were found on laboratory analysis. He was initially treated conservatively because of his old age. On the 12th day from admission, the patient showed drowsy mentality, an increase in the SDH on the CT, increased pressure on the left ventricle, and aggravation of the midline shift toward the right (Figure 2B). The CT showed a mixture of solid blood clots and fluid hematoma in the SDH. The tube was removed two days after the surgery based on a CT that showed sufficient removal of the hematoma (Figure 2C). The CT at postoperative day 7 showed no recurrence of the hematoma, and the GOS score was 5 at discharge (Figure 2D).
Due to the increase in the elderly population, the number of elderly patients with acute SDHs caused by minor head trauma is growing. In particular, a high prevalence of acute SDHs has been reported among elderly patients with hemorrhagic tendencies caused by antiplatelet agents or anticoagulants.714151719)
SDHs are divided into three stages: acute, subacute, and chronic. In terms of the findings on the form of SDHs on brain CT scans, those in the solid form of a blood clot are in the acute stage, those in a mixed form (a solid blood clot and fluid hematoma) are in the subacute stage, and those in the fluid form are in the chronic stage.520) In terms of time, acute SDHs occur within 72 hours of receiving an injury, SASDHs occur within 4 to 20 days of receiving an injury, and chronic SDHs occur within three weeks or longer of receiving an injury.6) Acute and chronic SDHs are easy to differentiate, but subacute and chronic SDHs are difficult to differentiate clearly.
When an acute SDH is small and there are no signs of increased intracranial pressure, conservative treatment is provided, whereas when a hematoma is large and the neurological condition is serious due to the increased intracranial pressure, it should be removed immediately via a craniotomy.1) A large chronic SDH over 1 cm thick requires surgical treatment, and unlike a solid acute hematoma, a chronic fluid hematoma can be treated by removing the hematoma using burr hole drainage.818) During conservative treatment of an acute SDH, the hematoma can increase suddenly in the subacute stage, requiring surgical treatment.1)
In the case of an SASDH with a mix of a solid blood clot and fluid, burr hole drainage can remove the fluid, but it is difficult to remove the solid blood clot.820) In particular, for a thick, widely distributed solid hematoma, craniotomy should be considered, as it can completely remove the hematoma, but it is associated with complications from the general anesthesia and the surgery itself. In particular, high-risk craniotomies can be difficult to perform on elderly patients with a high risk of complications from general anesthesia due to internal medicine diseases, such as heart or respiratory diseases, or on patients with a high risk of excessive blood loss due to the use of antiplatelet agents or anticoagulants that result in decreased hemostasis.1621) Hematoma drainage using a burr hole and urokinase is generally performed to decrease the intracranial pressure by draining the cerebrospinal fluid in spontaneous acute ventricular intracerebral hemorrhages and removing the solid hematoma.2311)
In cerebral hemorrhages associated with anticoagulants, large amounts of blood can be lost during surgery, and the risk of hemorrhage recurrence is high.13) In these patients, hematoma drainage using a burr hole and urokinase has been reported to be a good micro-invasive treatment, as it has a lower risk of mortality and complications than craniotomy.16) Good outcomes have been reported for hematoma drainage using a burr hole and urokinase in traumatic epidural hematomas with minor neurological abnormalities that do not require the reduction of the intracranial pressure by removing the hematoma through craniotomy.12)
Urokinase liquefied the solid hematoma that was difficult to drain and rapid SDH drainage was possible within 3 days, the patient's symptom was improved quickly and early ambulation was possible so this surgical treatment could reduce the length of hospital stay.
In this study, the patients' mean age was 73.3 years, and there was no mortality or morbidity in any of the patients. Therefore, it is thought that a subdural drain using urokinase after a burr hole hematoma evacuation is useful for elderly patients with SASDHs. However, our report is on a small number of cases, and it is a retrospective study. Thus, more cases with accumulated clinical experience are needed for the further analysis of reliability and outcomes. In addition, it will be necessary to evaluate the effectiveness and safety of our procedure compared with craniotomy for hematoma removal in elderly patients with SASDHs.
A subdural drain using urokinase after burr hole hematoma evacuation under local anesthesia is thought to be an effective and safe method of blood clot removal with low morbidity. This surgical method is less invasive for treating elderly patients with SASDHs. In particular, it may be a good alternative treatment to craniotomy for elderly patients with a high risk of complications from general anesthesia due to internal medicine diseases, such as heart or respiratory diseases, or for patients with a high risk of excessive blood loss due to the use of antiplatelet agents or anticoagulants.
References
1. Bullock MR, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW, et al. Surgical management of acute subdural hematomas. Neurosurgery. 2006; 58:S16–S24. PMID: 16710968.

2. Chen X, Chen W, Ma A, Wu X, Zheng J, Yu X, et al. Frameless stereotactic aspiration and subsequent fibrinolytic therapy for the treatment of spontaneous intracerebral haemorrhage. Br J Neurosurg. 2011; 25:369–375. PMID: 20874455.

3. Gaberel T, Montagne A, Lesept F, Gauberti M, Lemarchand E, Orset C, et al. Urokinase versus Alteplase for intraventricular hemorrhage fibrinolysis. Neuropharmacology. 2014; 85:158–165. PMID: 24846802.

4. Han SB, Choi SW, Song SH, Youm JY, Koh HS, Kim SH, et al. Prediction of chronic subdural hematoma in minor head trauma patients. Korean J Neurotrauma. 2014; 10:106–111. PMID: 27169043.

5. Izumihara A, Orita T, Tsurutani T, Kajiwara K. Natural course of non-operative cases of acute subdural hematoma: sequential computed tomographic study in the acute and subacute stages. No Shinkei Geka. 1997; 25:307–314. PMID: 9125713.
6. Izumihara A, Yamashita K, Murakami T. Acute subdural hematoma requiring surgery in the subacute or chronic stage. Neurol Med Chir (Tokyo). 2013; 53:323–328. PMID: 23708224.

7. Kawamata T, Takeshita M, Kubo O, Izawa M, Kagawa M, Takakura K. Management of intracranial hemorrhage associated with anticoagulant therapy. Surg Neurol. 1995; 44:438–442. discussion 443. PMID: 8629228.

8. Kenning TJ, Dalfino JC, German JW, Drazin D, Adamo MA. Analysis of the subdural evacuating port system for the treatment of subacute and chronic subdural hematomas. J Neurosurg. 2010; 113:1004–1010. PMID: 20509728.

9. Kuwahara S, Fukuoka M, Koan Y, Miyake H, Ono Y, Moriki A, et al. Diffusion-weighted imaging of traumatic subdural hematoma in the subacute stage. Neurol Med Chir (Tokyo). 2005; 45:464–469. PMID: 16195646.
10. Lind CR, Lind CJ, Mee EW. Reduction in the number of repeated operations for the treatment of subacute and chronic subdural hematomas by placement of subdural drains. J Neurosurg. 2003; 99:44–46.

11. Liu L, Shen H, Zhang F, Wang JH, Sun T, Lin ZG. Stereotactic aspiration and thrombolysis of spontaneous intracerebellar hemorrhage. Chin Med J (Engl). 2011; 124:1610–1615. PMID: 21740764.
12. Liu W, Ma L, Wen L, Shen F, Sheng H, Zhou B, et al. Drilling skull plus injection of urokinase in the treatment of epidural haematoma: a preliminary study. Brain Inj. 2008; 22:199–204. PMID: 18240049.

13. Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet. 2005; 365:387–397. PMID: 15680453.

14. Oertel M, Kelly DF, McArthur D, Boscardin WJ, Glenn TC, Lee JH, et al. Progressive hemorrhage after head trauma: predictors and consequences of the evolving injury. J Neurosurg. 2002; 96:109–116. PMID: 11794591.

15. Patel NY, Hoyt DB, Nakaji P, Marshall L, Holbrook T, Coimbra R, et al. patterns of failure of nonoperative management. J Trauma. 2000; 48:367–374. PMID: 10744271.
16. Rohde V, Uzma N, Rohde I, St Clair E, Samadani U. Fibrinolytic therapy versus craniotomy for anticoagulant-associated intracerebral hemorrhage. Clin Neurol Neurosurg. 2009; 111:518–522. PMID: 19297083.

17. Rosand J, Eckman MH, Knudsen KA, Singer DE, Greenberg SM. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med. 2004; 164:880–884. PMID: 15111374.

18. Singla A, Jacobsen WP, Yusupov IR, Carter DA. Subdural evacuating port system (SEPS)-minimally invasive approach to the management of chronic/subacute subdural hematomas. Clin Neurol Neurosurg. 2013; 115:425–431. PMID: 22763191.

19. Son S, Yoo CJ, Lee SG, Kim EY, Park CW, Kim WK. Natural course of initially non-operated cases of acute subdural hematoma: the risk factors of hematoma progression. J Korean Neurosurg Soc. 2013; 54:211–219. PMID: 24278650.
20. Takeuchi S, Takasato Y, Otani N, Miyawaki H, Masaoka H, Hayakawa T, et al. Subacute subdural hematoma. Acta Neurochir Suppl. 2013; 118:143–146. PMID: 23564121.

21. Vigué B, Ract C, Tremey B, Engrand N, Leblanc PE, Decaux A, et al. Ultra-rapid management of oral anticoagulant therapy-related surgical intracranial hemorrhage. Intensive Care Med. 2007; 33:721–725. PMID: 17260127.

FIGURE 1
Case 2 was a 71-year-old female patient who presented with headache after a traumatic head injury caused by a slip. (A) At admission, a computed tomography (CT) scan showed a hyperdense hematoma in the subdural space in the right frontotemporal convexity. (B) She had sudden onset left-sided hemiparesis at 17 days after admission. The follow-up CT showed a mixed hypodense and hyperdense subdural hematoma (SDH) and midline shift progression. (C) An immediately postoperative CT scan showed a catheter in the subdural space and a significant amount of residual hematoma with mass effect. (D) A CT scan at postoperative 7 days showed a marked decrease of the SDH and resolution of the mass effect.
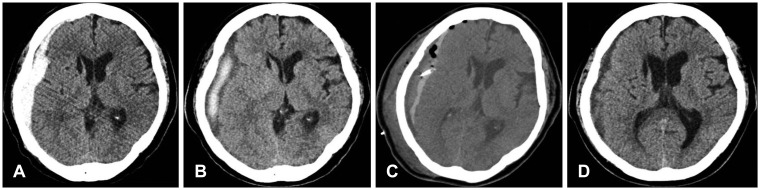
FIGURE 2
Case 6 was a 79-year-old male patient who presented with sensory aphasia and cognitive deterioration after a traumatic head injury caused by a slip. (A) At admission, a computed tomography (CT) scan showed a hyperdense hematoma in the subdural space in the left frontotemporal convexity. (B) He experienced aggravation of his symptoms at 12 days after admission. The follow-up CT showed a mixed hypodense and hyperdense subdural hematoma (SDH) and midline shift progression. (C) An immediately postoperative CT scan showed a catheter in the subdural space and the partial removal of the hematoma. (D) A CT scan at postoperative 7 days showed a marked decrease of the SDH and resolution of the mass effect.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download