The effectiveness of DC as a treatment approach to refractory intracranial hypertension, a consequence of failed medical treatment of traumatic brain injury or an intracranial vascular lesion, has been reported by several studies.
1121425262729) Bor-Seng-Shu et al.
4) reported their review article related to the contribution of DC in reducing ICP and increasing CPP among patients with refractory intracranial hypertension. They suggested that DC seems to be an effective treatment for reducing ICP and increasing CPP. Yamakami and Yamaura
27) also reported a result of similar effects of DC with respect to increasing CPP. On the other hand, it was also reported that DC could exacerbate it by increasing focal cerebral blood flow, which might cause brain swelling.
4102327) In addition, other post-DC complications may include: 1) perioperative complications such as expansion of contusion, new subdural and epidural hematomas contralateral to the decompressed hemisphere, and external cerebral herniation; and 2) delayed complications, such as subdural hygroma or wound infection.
23) In the aspect of general brain protection and cosmesis, except for special cased, cranioplasty is the procedure indicated for almost all cases following DC.
15) More recent reports have suggested that the rationale behind the performance of cranioplasty is not just a cosmetic matter but also therapeutic, through which cranioplasty helps to optimize neurological recovery.
2391015212226) Fodstad et al.
9) asserted in their report of 40 cases of cranioplasty that hydrodynamic changes of CSF before and after cranioplasty, including resting pressure, sagittal sinus pressure, buffer volume, elastance at resting pressure and pulse variations at resting pressure, are closely related to improvement of their symptoms after cranioplasty. Regardless, there have been quite a few literary considerations on various complications arising from post-DC cranioplasty.
10131530) Gooch et al.
10) analyzed the complications of cranioplasty following DC in their study of 62 cases of cranioplasty. They summarized postoperative wound infection, intracranial hematoma, bone resorption, sunken bone plate, intraoperative hemodynamic instability, deep vein thrombosis, etc. as complications that might develop after cranioplasty. The optimal time for cranioplasty after DC has not been determined yet. Previously, it is generally accepted that cranioplasty is carried out in 3 months after DC, but the procedures is performed 6 months after DC in cases of intracranial infection.
20) Recently, however, there are reports that early cranioplasty of 5 to 8 weeks after DC can further facilitate neurologic recovery and also lead to less postoperative complications.
1520) It is known that the frequency of hydrocephalus occurring in patients with large craniectomy is about 10 to 40%.
11428) Pachatouridis et al.
18) suggested that development of hydrocephalus after DC has been attributed to focal destructive lesions or ischemic insults, that lead to neuronal loss and severe atrophy of brain parenchyma, adhesive arachnoiditis of the basal cisterns, blood blocking and dysfunction of the arachnoid granulations, and gradient between the atmospheric pressure and ICP, which leads to inward displacement of the scalp and decrease of CSF flow over the convexity. In patients with a cranial defect and a CSF-circulation disorder, both cranioplasty and VP shunt operation may be necessary. It is known that performing two operations simultaneously has a higher risk of complications than performing them one at a time in staged operations.
1318) Pachatouridis et al.
18) also report that cranioplasty and ventriculostomy followed by a second stage placement of a VP shunt are associated with fewer complications in the treatment of hydrocephalus after DC. Oh et al.
17) asserted in their report that the outcome of shunt operation after cranioplasty tends to be better than cranioplasty after shunt operation for patients with a large cranial defect and hydrocephalus. In patients with a severely depressed scalp flap after DC and VP shunt, there is a potential risk of developing an intracranial hematoma as a complication in performing cranioplasty. Liao and Kao
16) tried to eliminate the dead space between the skull plate and the dura, and lessen the risk of complications related to intracranial hematoma that occurred secondary to cranioplasty. They suggested a method to minimize the dead space by inducing an expansion of the depressed brain. This was achieved by creating a temporary occlusion with an aneurysm clip in the distal catheter of the previously inserted VP shunt before they undertook cranioplasty. On the other hand, some authors reported that hydrocephalus in combination with a cranial defect can be generally treated with one-stage operations.
730) Carvi and Höllerhage
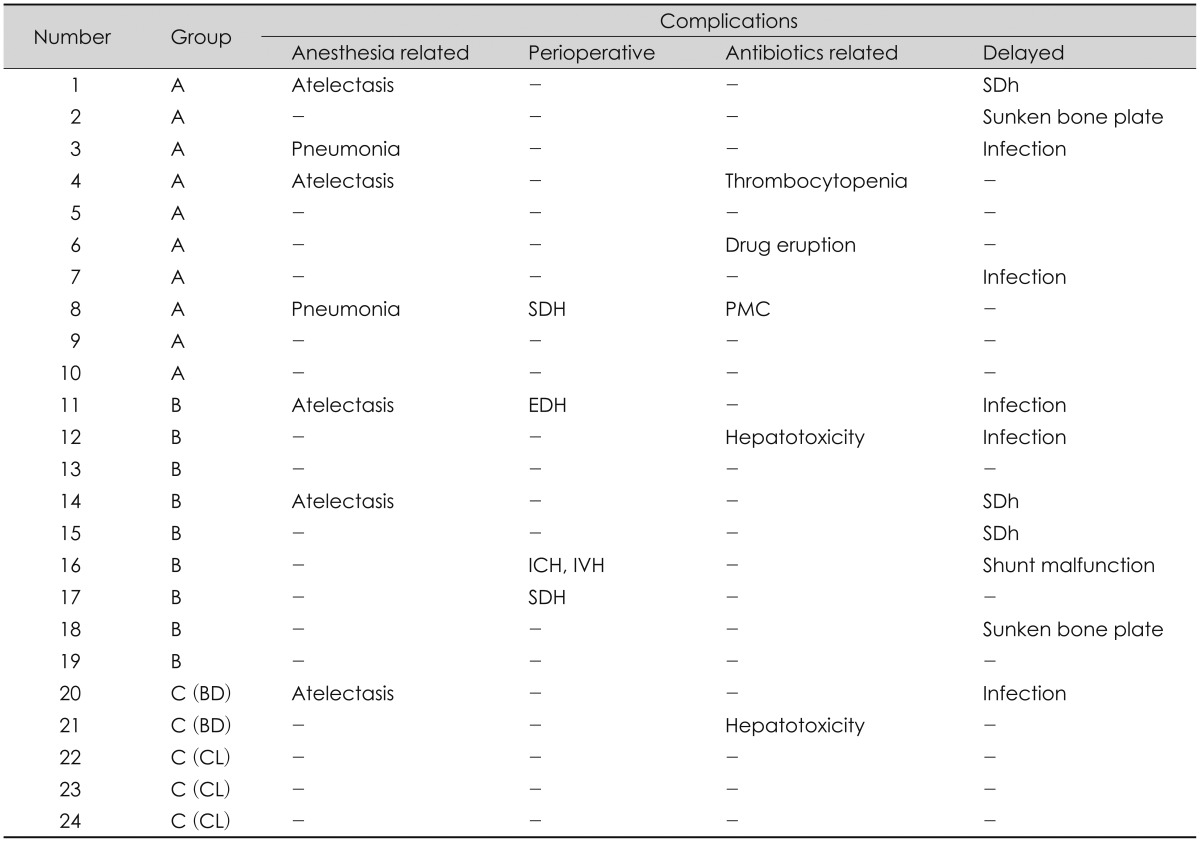
7) reported that cranioplasty combined with implementation of a shunt system allowed ICP changes to adjust dynamically after the procedures. The combined procedure reduced the number of required surgical procedures and complications. This study reviewed cases of one-stage operation of cranioplasty and shunt implantation, and cases of staged operation where each procedure was performed one at a time. Their complications were retrospectively examined and attempts were made to reduce complications that could develop in each. Complications of staged operation were associated with the number of general anesthesia and operation. Thus, both procedures were combined into a one-stage simultaneous operation to reduce these numbers. The complications, such as intracranial hematoma and subdural hygroma, which develop often in a one-stage operation, were prevented by blocking the CSF drainage (temporary occlusion of the distal shunt catheter). This would avert the sunken down effect of the brain. With respect to complications, such as intracranial hematoma or subdural hygroma that develops often in a one-stage operation, the assumption was that the risk of complications of one-stage operation could be reduced by preventing the sunken down effect of the brain through blocking CSF drainage, and thus inducing adhesion between the dura mater and cranium after cranioplasty. In an attempt to prevent neuronal damage secondary to hydrocephalus that might develop in this period, an ICP monitor-ing catheter was inserted during surgery. The plan was to aspirate CSF from the reservoir bag of the VP shunt system as needed, but there was no increased ICP to the extent of requiring aspiration in this study. The occlusion device could easily be removed by a simple procedure at bedside, and there was no particular effect on the wall structure of the shunt catheter as shown in observations. The shunt valve pressure was initially set at 200 mm Hg, and was gradually decreased as patients were followed up with brain CT. The results were satisfactory and no other complication was observed, except for a mild wound infection that was pharmacologically treated.
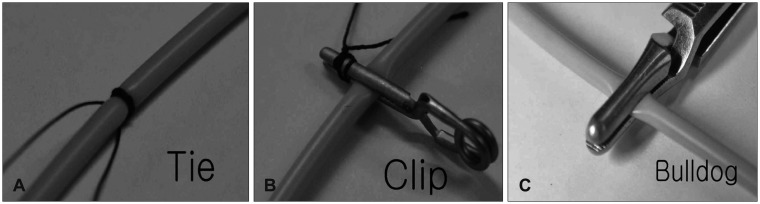
Despite these many positives, some limitations to our conclusions exist. One variable that could affect the nature of our results would be the selection bias such as surgeon related complications and indications in application of a Yasargil clip or a Bulldog clamp. Our study was not designed to clarify such limitations. Furthermore, the number of patients in Group C was too small to be a statistical analysis. So the results of this study was not enough to generalize. Therefore, future studies should be performed with a large number in Group C to validate these results. Prospective studies are further planned to confirm the effects of these procedures in patients with large cranial defects and CSFcirculation disorder. Further long-term follow-up studies with a large number of patients are necessary.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download