Abstract
Objective
Brain atrophy and subdural hygroma were well known factors that enlarge the subdural space, which induced formation of chronic subdural hematoma (CSDH). Thus, we identified the subdural volume that could be used to predict the rate of future CSDH after head trauma using a computed tomography (CT) volumetric analysis.
Methods
A single institution case-control study was conducted involving 1,186 patients who visited our hospital after head trauma from January 1, 2010 to December 31, 2014. Fifty-one patients with delayed CSDH were identified, and 50 patients with age and sex matched for control. Intracranial volume (ICV), the brain parenchyme, and the subdural space were segmented using CT image-based software. To adjust for variations in head size, volume ratios were assessed as a percentage of ICV [brain volume index (BVI), subdural volume index (SVI)]. The maximum depth of the subdural space on both sides was used to estimate the SVI.
Results
Before adjusting for cranium size, brain volume tended to be smaller, and subdural space volume was significantly larger in the CSDH group (p=0.138, p=0.021, respectively). The BVI and SVI were significantly different (p=0.003, p=0.001, respectively). SVI [area under the curve (AUC), 77.3%; p=0.008] was a more reliable technique for predicting CSDH than BVI (AUC, 68.1%; p=0.001). Bilateral subdural depth (sum of subdural depth on both sides) increased linearly with SVI (p<0.0001).
Chronic subdural hematoma (CSDH) is a relatively common disease encountered in neurosurgical practice, occurring at a rate of 1-2 per 100,000/year. With advanced imaging technologies, such as computed tomography (CT) and magnetic resonance image (MRI), many studies have been conducted to understand the pathophysiological mechanisms, progress, recurrence, and predisposing factors for CSDH, but pathogenesis remains controversial.10) Known risk factors for CSDH are trauma, old age, alcohol addiction, epilepsy, a blood coagulation disorder, anticoagulant use, brain atrophy, and subdural hygroma. Brain atrophy and subdural hygroma expand the subdural space, which increases the frequency of CSDH occurrence. However, studies analyzing the degree of subdural space expansion and its association with the frequency of CSDH are insufficient. In particular, almost no studies have quantified volume of the subdural space and the risk for CSDH. Therefore, in this study, we measured subdural space volume in patients diagnosed with CSDH after head trauma on subsequent CT scans using a volumetric measurement method and investigated the effects of the subdural space on the occurrence of CSDH.
A total of 1,186 patients visited the emergency room and outpatient clinic at our hospital after head injury from January 2010 to December 2014. Patients, who had undergone surgery for CSDH in time of visiting the hospital (n=179), patients in whom surgery was performed within 7 days due to hemorrhage aggravation (n=62) and patients with initial Glasgow Coma Scale (GCS) score <12 points (n=73) were excluded from the study. A retrospective study was carried out on 872 patients. Among the 872 patients, CSDH necessitating surgical treatment was identified in 51 patients (CSDH group). Age and sex matched 50 patients were selected as a control group among the 821 patients using the randomization function in the Excel program to minimize the effects of other risk factors except subdural space volume, and a comparative analysis was performed on 101 patients.
Intracranial volume (ICV), and cerebral hemisphere and subdural spaces were measured using sequential volume mapping in the Gamma-Plan program ver. 10.1 (Elekta Instruments AB, Stockholm, Sweden). Infra-tentorial volume was excluded. Two neurosurgery residents and one neuro-radiologist measured cross-sectional volume on CT scans performed on patients during hospitalization without any preliminary information on the patient, and the mean of each measured value was calculated. To reflect differences in ICV between patients, ratios between ICV and the brain and subdural space volume were measured, and were respectively designated as the brain volume index (BVI) and subdural volume index (SVI). In addition, receiver operating characteristic (ROC) curve analysis was conducted to evaluate utility and cut-off value between the two indices. Three appraisers analyzed the initial CT scans of all patients, and maximum depth of the subdural space was measured on both sides as an indirect measure of subdural space volume. The larger of the two measured values was called unilateral subdural depth, and the sum of subdural space depth on both sides was called bilateral subdural depth.
Statistical analysis was carried out with SPSS version 20.0 for Windows software (SPSS Inc., Chicago, IL, USA). The t-test and chi-square test were utilized, and a p-value <0.05 was considered significant.
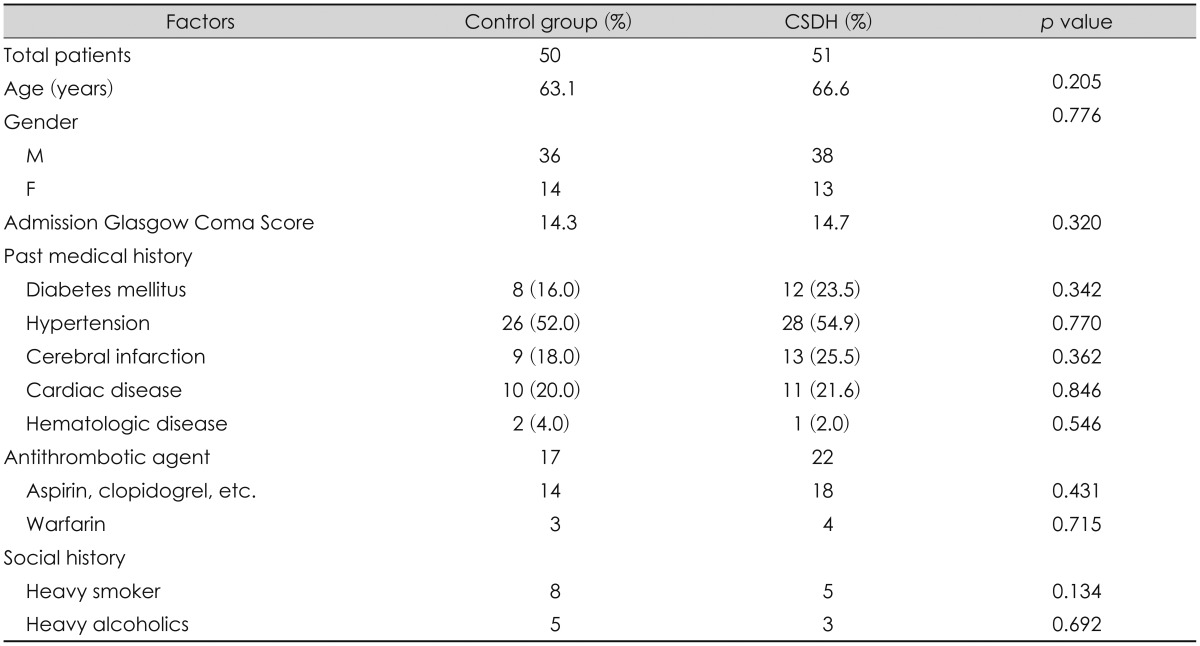
The mean age of all participants was 64.8 years (range, 17 to 87 years). Mean ages of the control and CSDH groups were 63.1 and 66.6 years, respectively (p=0.205). There were 36 male patients and 14 female patients in the control group, and 38 male patients and 13 female patients in the CSDH patient group (p=0.776). Mean GCS scores at the first visit were 14.3 and 14.7 in the control and CSDH groups, respectively. No differences in known risk factors or medication and social history were observed between the two groups (Table 1).
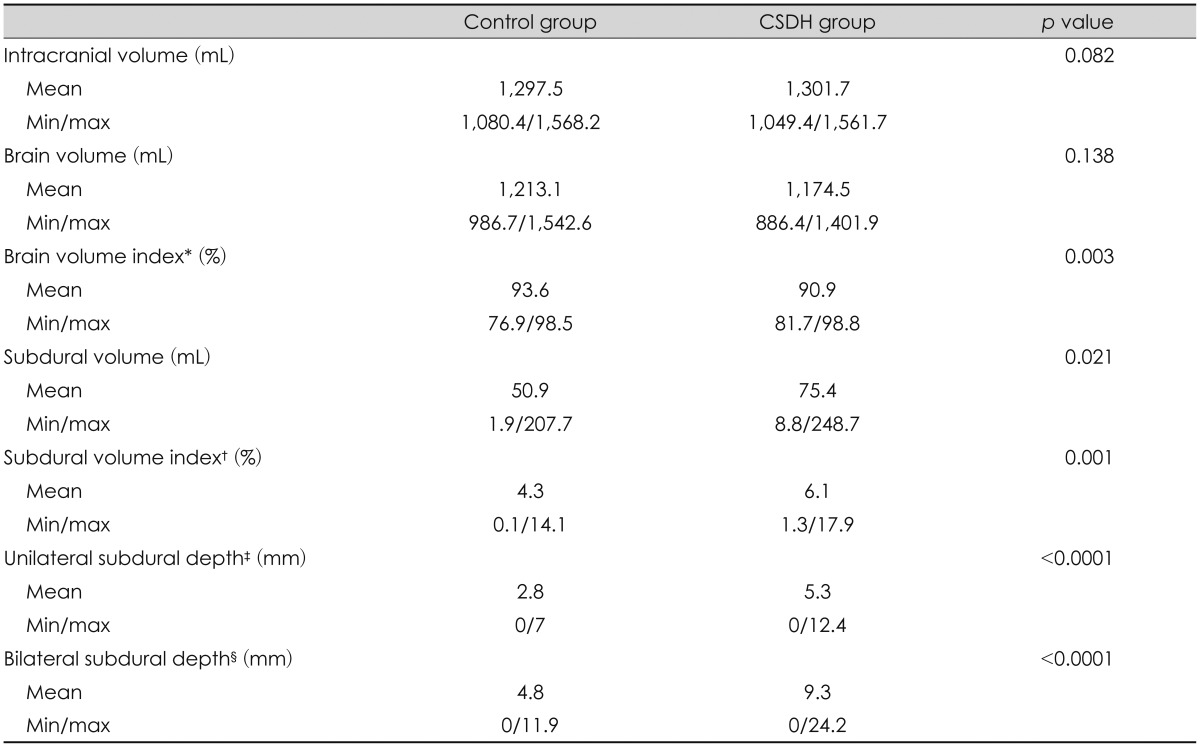
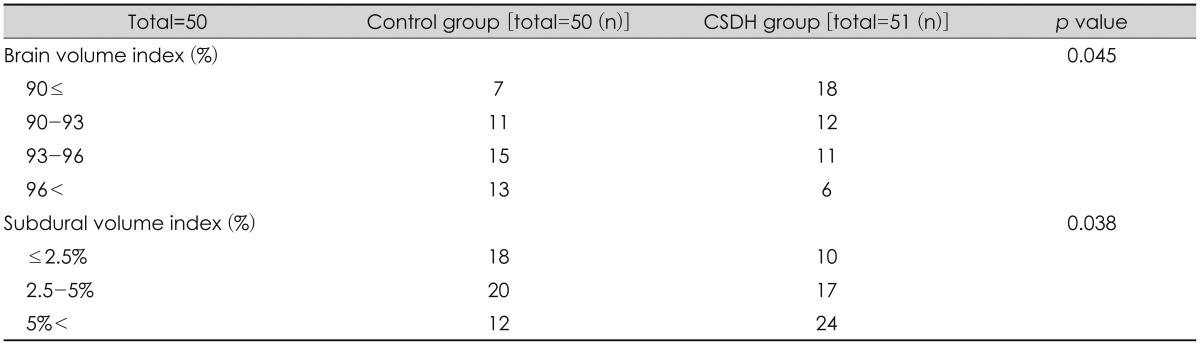
Mean ICV in the control group was 1,297.5 mL, and that in the CSDH group was 1,301.7 mL. Mean cerebral hemisphere volumes were 1,213.2 mL and 1,174.5 mL in the control and CSDH groups, respectively. No differences in intracranial or cerebral volume were detected between two groups. However, the BVI was significantly different between two groups (93.6% vs. 90.9%, p=0.003) (Table 2). When the BVI was divided into 3% intervals, a significant result was observed between SVI and occurrence of CSDH (Table 3). Cut-off value of BVI was 94.8% (sensitivity, 82.9% and specificity, 47.8%), and the area under the curve (AUC) was 68.1% (poor) (Figure 1).
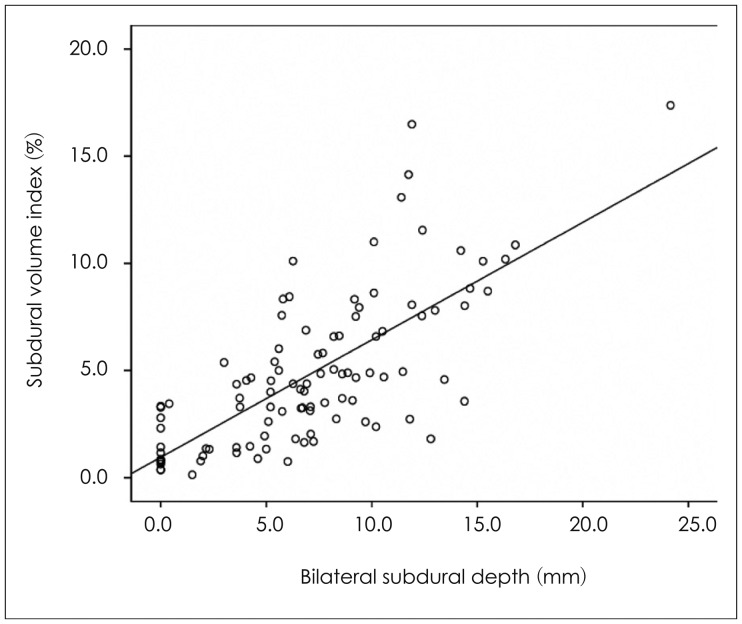
Unlike brain hemispheric volume, subdural space volume differed between two groups (50.9 mL vs. 75.4 mL) (p= 0.021). The mean SVI was 4.3% in the control group and 6.1% in the CSDH group (p=0.001). When the SVI was divided into 2.5% intervals, a significant result was observed between the SVI and the occurrence of CSDH (Table 3). Cut-off value of SVI was 6.6% (sensitivity, 37.2% and specificity, 86%), and the AUC was 77.3% (fair) (Figure 1). Among the overall patient characteristics, age and the SVI were moderately positively correlated (R=0.57, p<0.0001), and coefficient of determination was 56.8% (Figure 2).
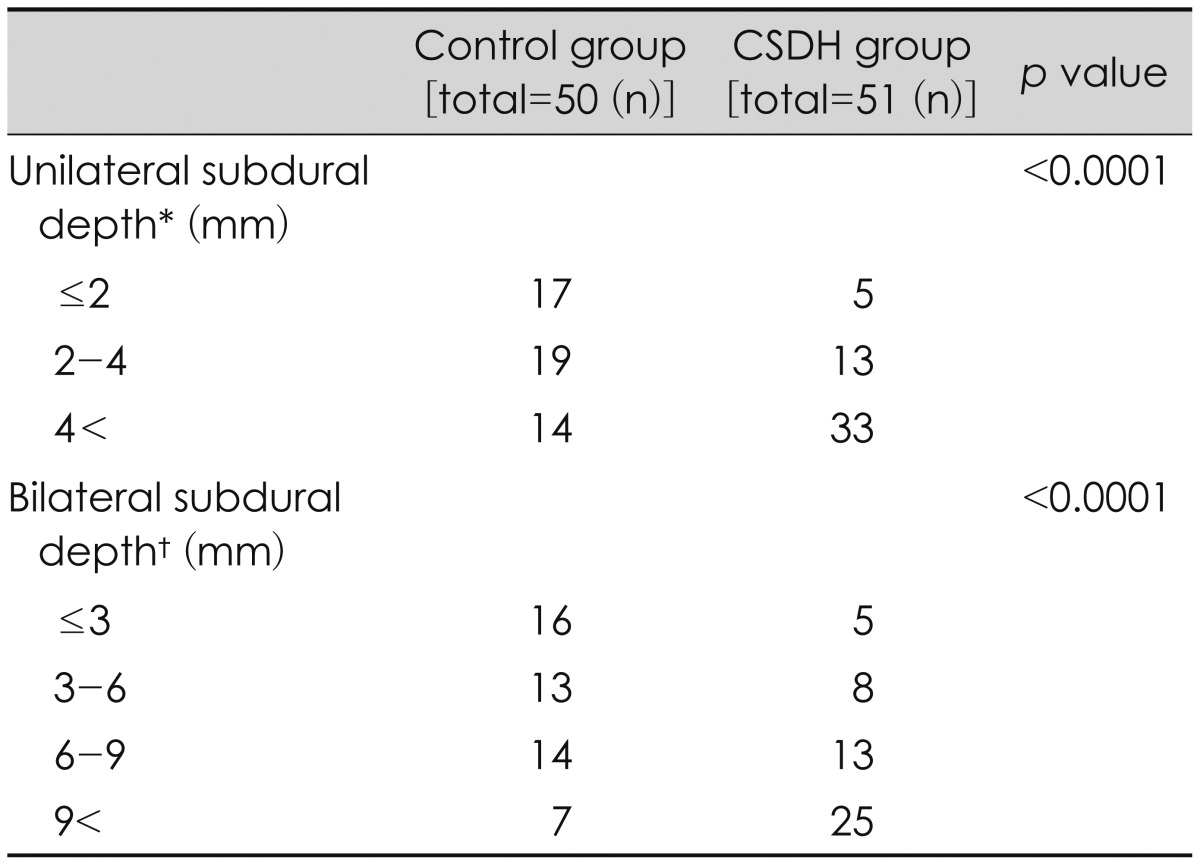
Mean unilateral subdural depth was 2.8 mm in the control group and 5.3 mm in the CSDH group. Mean bilateral subdural depth was 4.8 mm in the control group and 9.3 mm in the CSDH group (p<0.0001, p<0.0001) (Table 2). When unilateral subdural depth was divided into 2 mm intervals and the bilateral subdural depth was divided into 3 mm intervals, significantly different results were seen between each group and the occurrence of CSDH (Table 4). Cut-off value of the unilateral subdural depth in the CSDH group was 4.5 mm, and cut-off value of the bilateral subdural depth in the CSDH group was 6.2 mm. The AUC values were 67% and 77.2%, respectively. The unilateral and bilateral subdural space depth values were moderately correlated with the SVI (R=0.68, R=0.72, p<0.0001), and the coefficient of determination was R2=0.47 for unilateral and R2=0.52 for bilateral subdural depth, respectively.
Approximately one-third of patients with CSDH have a history of minor trauma, and risk factors other than brain atrophy and subdural hygroma are old age, chronic alcohol addiction, blood coagulation disorders, anticoagulant use, and epilepsy.23) In this study, 50 age and sex-matched patients were randomly selected as the control group to reduce the effect of other factors on CSDH except subdural space volume. Thus, the baseline clinical characteristics were not different between the two groups, which can be considered appropriate random sampling. As a result of age and gender matched random sampling, baseline clinical characteristics were not different between two groups, and this can be considered as performance of random sampling in an appropriate manner.
With various methods for the brain volumetric analysis using CT and MRI, many international studies have been conducted on the brain atrophy caused by alcoholism or Alzheimer's disease, and other aging phenomena.57) In domestic studies, image analysis of brain atrophy was mainly evaluated by the cross-sectional image or using simple distance measuring method, however, few studies have measured the brain volume to assess the CSDH or identify the level of progression.
Ge et al.5) studied changes in brain volume according to age in 54 adults by MRI, and reported that brain volume was 1,071.4 mL in a group with mean age of 67 years (≤50 years), and 1,217.7 mL in a group with mean age of 32 years (<50 years). In our study, mean brain volume of 101 patients was 1,178.2 mL, 1,193.6 mL for those 50 years or higher, and 1,268.1 mL in those less than 50 years. As head sizes differed in each individual patient, it was difficult to determine the actual level of brain atrophy using only brain hemispheric volume. Therefore, we compared the BVI between 2 groups; 90.9% of CSDH group and 93.6% of control group with statistical significance (p=0.003) (Table 2).
Post-traumatic subdural fluid collection is observed in 6% of thses patients, and is known to expand the subdural space and accelerate formation of an outer membrane in the subdural space.49) This outer membrane forms new internal capillaries and sinusoidal blood vessels that easily penetrate the plasma component to increase the subdural space.8) In addition, repetitive bleeding occurs along with regression of capillary endotheliocyte, which precedes CSDH.410) We found that subdural space volume was larger in the CSDH group than in the control group. We conducted comparative analysis of SVI to reflect individual head size, and detected difference between CSDH group and the control group.
In this study, ROC curve was used to assess the utility and cut-off value of BVI and SVI. As a result, SVI was a better measure to reflect occurrence of CSDH. Considering the sensitivity and the specificity of BVI and SVI, brain atrophy expands the subdural space but actual occurrence of CSDH requires more than specific level of subdural space expansion.
Measuring brain and subdural space volumes increases time and effort consumption realistically, and requires a complex process, therefore, it is difficult to be applied to all patients during an actual clinical trial. Ambarki et al.1) volumetrically analyzed brain atrophy and brain volume changes by MRI and compared traditional linear measures such as the Evans index (EI) and frontal and occipital horn ratio (FOHR). Both EI and FOHR showed strong quantitative linear relationships, but the prediction interval was too large, and it was difficult to reflect brain volume and atrophy using only a traditional linear measures. Gebel et al.6) applied the existing ABC/2 formula to patients with subdural hemorrhage, indirectly measured intracerebral hemorrhage volume, and compared it with a volumetric analytical value. They reported that the modified ABC/2 formula had a very high quantitative linear relationship with actual volume. In this study, the maximum depths of both the subdural spaces were measured to indirectly reflect the SVI, and both showed moderate linear relationship with the SVI in a simple correlation analysis. However, it was possible to moderately predict (fair grade) the occurrence of CSDH using only the sum of the bilateral subdural depth values; therefore, it is expected to be easily applicable to an actual clinical study.
The limitations of this study are that it was a retrospective analysis, and characteristics and volumes of the 821 patients were not compared with the CSDH group due to a time limitation.
However, despite this limitation, this study was significant with respect to the quantitative analysis of the relationship between CSDH, brain atrophy, and subdural space. A regression analysis and prospective cohort study should be conducted in the future considering other risk factors.
We quantitatively analyzed the relationship between CSDH, brain atrophy, and the subdural space. Brain atrophy plays a role in the occurrence of CSDH. The SVI more reliably predicted the occurrence of CSDH than that of BVI, which reflected brain atrophy. The sum of subdural depth on both sides can be used to predict the occurrence of CSDH in clinical practice.
References
1. Ambarki K, Israelsson H, Wåhlin A, Birgander R, Eklund A, Malm J. Brain ventricular size in healthy elderly: comparison between Evans index and volume measurement. Neurosurgery. 2010; 67:94–99. discussion 99PMID: 20559096.
2. Aspegren OP, Åstrand R, Lundgren MI, Romner B. Anticoagulation therapy a risk factor for the development of chronic subdural hematoma. Clin Neurol Neurosurg. 2013; 115:981–984. PMID: 23128014.

3. Baechli H, Nordmann A, Bucher HC, Gratzl O. Demographics and prevalent risk factors of chronic subdural haematoma: results of a large single-center cohort study. Neurosurg Rev. 2004; 27:263–266. PMID: 15148652.

4. Camel M, Grubb RL Jr. Treatment of chronic subdural hematoma by twist-drill craniotomy with continuous catheter drainage. J Neurosurg. 1986; 65:183–187. PMID: 3723175.
5. Ge Y, Grossman RI, Babb JS, Rabin ML, Mannon LJ, Kolson DL. Age-related total gray matter and white matter changes in normal adult brain. Part I: volumetric MR imaging analysis. AJNR Am J Neuroradiol. 2002; 23:1327–1333. PMID: 12223373.
6. Gebel JM, Sila CA, Sloan MA, Granger CB, Weisenberger JP, Green CL, et al. Comparison of the ABC/2 estimation technique to computer-assisted volumetric analysis of intraparenchymal and subdural hematomas complicating the GUSTO-1 trial. Stroke. 1998; 29:1799–1801. PMID: 9731597.

7. Goldszal AF, Davatzikos C, Pham DL, Yan MX, Bryan RN, Resnick SM. An image-processing system for qualitative and quantitative volumetric analysis of brain images. J Comput Assist Tomogr. 1998; 22:827–837. PMID: 9754125.

8. Mori K, Mitsuoka H, Cho K, Tajima A, Maeda M. Rate constant of gadolinium (Gd)-DTPA transfer into chronic subdural hematomas. Neurol Res. 1996; 18:126–134. PMID: 9162866.

9. Sato S, Suzuki J. Ultrastructural observations of the capsule of chronic subdural hematoma in various clinical stages. J Neurosurg. 1975; 43:569–578. PMID: 1181389.

10. Winn HR. Youmans neurological surgery. ed 5. Philadelphia: WB Saunders;2004.
FIGURE 1
Relationship between the subdural volume index (SVI) and brain volume index (BVI) in the control and chronic subdural hematoma (CSDH) groups. Cumulative distribution of BVI and SVI between the control and CSDH groups. The cut-off value of SVI was 6.6% (sensitivity, 37.2% and specificity, 86%) with area under the curve (AUC) of 77.3%. The cut-off value of BVI was 94.8% (sensitivity, 82.9% and specificity, 47.8%) with AUC of 68.1%. Note that CSDH group was markedly higher than SVI and BVI cut-off values.

FIGURE 2
Relation between the subdural volume index (SVI) and bilateral subdural depth. The relationship between the SVI and bilateral subdural depth. The correlation coefficient was 0.72 (p<0.0001), indicating that the SVI increases as bilateral subdural depth increases.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download