Abstract
Objective
Chronic subdural hematoma (CSDH), a disease commonly encountered by neurosurgeons, is treated by burr hole drainage (BHD). However, the optimal surgical technique among the three types of BHD has not been determined.
Methods
We conducted a retrospective study on BHD performed on 93 patients who were diagnosed with CSDH. The subjects were divided into three groups based on the surgical technique performed: single BHD without irrigation (Group A, n=31), double BHD without irrigation (Group B, n=32), and double BHD with irrigation (Group C, n=30). The clinical factors, radiological factors and recurrences were compared between the three groups. Moreover, independent factors affecting the recurrence were analyzed.
Results
The change in hematoma thickness was 29.77±7.94%, 49.73±12.87%, and 75.29±4.32% for Group A, B, and C, respectively, while the change in midline shift was 40.81±15.47%, 51.78±10.94%, and 56.16±16.16%, respectively. Thus, Group C showed the most effective for resolution of hematoma and midline shift (p<0.05). Group A, B, and C had 12 cases (38.7%), 8 cases (25.0%), and 3 cases (10.0%) of recurrences, respectively. Group C had a statistically significantly fewer recurrence rate than Group A (p<0.05). Double burr hole, irrigation, and coagulopathy were each identified as independent factors that reduce recurrence (p<0.05).
Chronic subdural hematoma (CSDH) is a disease commonly encountered by neurosurgeons.22) The occurrence of CSDH in elderly patients is 3 in 100,000.4) The prognosis of CSDH is reported to be relatively good; still, its recurrence rate is reported to be between 3 to 34%.314151725) Although various factors are related to the recurrence of CSDH, slightly contradicting results have been reported in different studies.2132023)
Surgery is considered the treatment of choice for CSDH; the surgical techniques include burr hole drainage (BHD), twisted craniotomy, and craniotomy. A recent study reported that BHD shows the best treatment outcome and has the fewest complications among the three techniques.30) BHD is performed in three different ways: single BHD without saline irrigation, double BHD without saline irrigation, and double BHD with saline irrigation. Although several studies comparing the outcomes of single BHD without saline irrigation and double BHD without saline irrigation have been reported, the results regarding the superiority of one over the other have been contradictory.122128) Moreover, even in studies comparing BHD with or without saline irrigation, the results were unclear.562032) Therefore, the optimal surgical treatment is still controversial. To the best of our knowledge, no study to date has compared all three techniques all together.
Accordingly, the objective of this study was to simultaneously compare the postoperative clinical and radiological outcomes of three surgical techniques for CSDH, along with the postoperative recurrence rate, and to determine the most effective technique for reducing postoperative recurrence.
The present study was a retrospective review of patients who had underwent BHD following diagnosis of CSDH between January 2010 and June 2014. In the surgical findings, CSDH was defined as hematoma wrapped in a thin capsule and consisting of dark reddish liquefied blood.19) A total of 123 cases of BHD were performed at our institution, of which, 93 cases were selected for the study. The remaining 30 cases were excluded because they involved a prior history of treatment for cerebral infarction or cerebral hemorrhage such as subarachnoid hemorrhage, or they received ventriculo-peritoneal shunt surgery, or due to a lack of follow-up for a minimum of 3 months. Comparisons were made after dividing the patients into three groups based on the surgical technique: single BHD without saline irrigation (Group A, n=31), single BHD without saline irrigation (Group B, n=32), and double BHD with saline irrigation (Group C, n=30). In each cases, surgical techniques were selected according to operator's preferred method.
In all patients, surgery was performed under general anesthesia. A high-speed drill was used to create the burr hole in all patients, after which, the dura was opened carefully to verify the CSDH capsule. In Group A, a single hole was drilled at the parietal boss and a 5N silicon catheter was inserted to perform the drainage, without saline irrigation. In Group B, 2 holes were drilled, one each at the parietal boss and frontal area, to each of which, a catheter was inserted in a mutually crossing direction, again without saline irrigation. In Group C, 2 holes were drilled and irrigation was performed thoroughly with saline to confirm the complete removal of hematoma. Next, 2 catheters were placed in the same manner as in Group B. In all patients, catheters were connected to drainage bags and fixed at the same height as the patient's tragus to allow continuous drainage. The catheters were removed when the patient showed improvement in neurologic symptoms, and when it was determined from computed tomography (CT) that sufficient drainage of the hematoma had occurred. CT exams were performed immediately after surgery, and 1 week and 3 months after surgery for follow up monitoring.
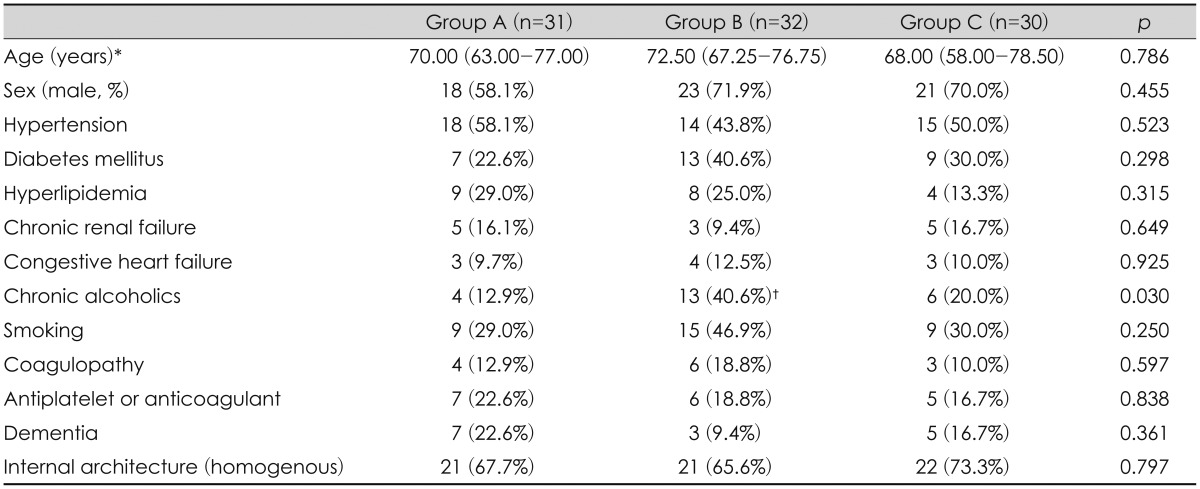
For comparisons of the three groups, age, gender, status of hypertension, diabetes, hyperlipidemia, chronic renal or heart insufficiency, chronic alcoholism, smoking, and coagulopathy [international normalized ratio (INR)14) >1.20], taking of anti-platelets or anti-coagulants, presence of dementia, and preoperative Glasgow Coma Scale29) were investigated via a chart review. For comparisons of clinical results from each group, hospital days, recurrence and mortality rates, and Glasgow Outcome Scale (GOS) at discharge8) were investigated. From the GOS results, good recovery and moderate disability were defined as good outcomes, whereas, severe disability, persistent vegetative state, and death were defined as poor outcomes.691629)
Recurrence in patients was evaluated by performing CTs prior to surgery and 1 day, 1 week, and 3 months after surgery. In the preoperative CT, the density of hematoma was measured, with uniform density being defined as homogenous internal architecture and mixed density being defined as heterogeneous internal architecture (Figure 1).1011) The resolution of hematoma was compared by calculating the change in thickness and midline shift of the hematoma from the area with the maximal amount of hematoma seen on CTs taken prior to and 1-day after surgery.26) The change was defined as a percentile obtained by dividing the preoperative CT value by the difference derived from subtracting the postoperative CT value from the preoperative CT value.
Recurrence of CSDH was defined as an increase in the amount of hematoma in the surgical area, change in hematoma density, or effacement of cerebral sulci seen on CT exam within 3 months after surgery.119) Revision surgery was performed when neurologic symptoms was occurred, such as mental decrease, motor weakness, and dysarthria, clearly accompanied the recurrence.
For intergroup comparisons, the distribution of the continuous data was first evaluated for normality using the Shapiro-Wilk test. The normally distributed data was presented as the mean±standard deviation and the groups were compared using analysis of variance and Tukey test as a post hoc test. The non-normally distributed data was expressed in median (Q1 to Q3) and this data was analyzed via the Kruskal-Wallis test followed by Bonferroni's correction. Descriptive variables were subjected to χ2 analysis or Fisher's exact test, as appropriate. Unadjusted logistic models were used to identify significant variables affecting the recurrence (Table 1).
Variables with p<0.10 were included in a multivariate logistic regression with conditional backward selection model. The level of statistical significance was set at p<0.05. All analysis was performed using SPSS 21.0 (IBM Corp., Armonk, NY, USA).
The baseline patient characteristics between Groups A, B, and C were compared. Age, gender, status of hypertension, diabetes, hyperlipidemia, renal insufficiency, heart insufficiency, smoking, and coagulopathy, taking of anti-platelets or anti-coagulants, and presence of dementia were similar between the three groups (Table 2). The number of chronic alcoholics was statistically significantly higher in Group B (p<0.05). The internal architecture on preoperative CT showed no statistically significant difference between the groups (Table 2).
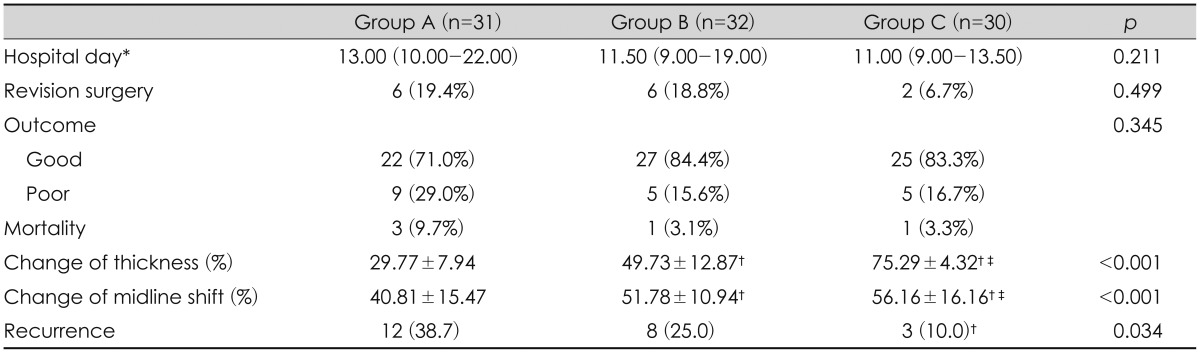
Postoperative clinical outcomes between the three groups were compared. When hospital days and outcome at discharge (GOS) between the three groups were compared, no statistically significant differences were found. The number of cases in which revision surgery was performed was 6 (19.4%), 6 (18.8%), and 2 (6.7%) in Groups A, B, and C, respectively. Thus, Group C had relatively fewer revision surgeries. The number of mortality cases was found to be 3 (9.7%), 1 (3.1%), and 1 (3.3%) in Groups A, B, and C, respectively (Table 3). The causes of death were founded as following: there were 2 cases of ventilator-associated pneumonia (VAP) and 1 case of catheter-induced sepsis (Group A), 1 case of VAP (Group B), and 1 case of unknown origin sepsis were investigated (Group C).
Change in hematoma thickness in Groups A, B, and C was 29.77±7.94%, 49.73±12.87%, and 75.29±4.32%, respectively, while change in midline shift was 40.81±15.47%, 51.78±10.94%, and 56.16±16.16%, respectively. Thus, Group C had the most effective for resolution of hematoma and midline shift (p<0.05). As for recurrence, there were 12 cases (38.7%) in Group A, 8 cases (25.0%) in Group B, and 3 cases (10.0%) in Group C. Thus, Group C had a statistically significantly lower recurrence rate than Group A (p<0.05) (Table 3).
The authors analyzed factors associated with the postoperative recurrence of CSDH by multivariate logistic regression analysis, and found that presence of coagulopathy, single burr hole and without saline irrigation were significantly independent risk factors associated with the recurrence of chronic subdural hematoma (p<0.05). Therefore, double BHD with saline irrigation is strong associated with reduction of postoperative recurrence (Table 4).
CSDH is a common disease,22) and the first choice treatment is surgery, which includes BHD, twisted craniotomy, and craniotomy. Among these, BHD is reported to have the best treatment outcome; however, the optimal surgical technique of BHD is still controversial. Therefore, we compared the postoperative clinical and surgical outcomes of the three surgical techniques, along with recurrence, to determine the technique most effective in preventing the recurrence of CSDH at same time.
Taussky et al.28) compared 63 cases of double BHD and 34 cases of single BHD, out of 97 cases of hematoma, and reported that the single burr hole group showed a higher recurrence rate. Ishibashi et al.6) divided patients who had undergone BHD for CSDH into two groups, based on whether irrigation was performed; it was reported that the group that underwent irrigation had better outcomes. Our study also showed that when simple comparisons of the radiologic results were made, the recurrence rate was statistically significantly lower in Group C than in the other two groups. For verification, a multivariate logistic regression analysis was performed with recurrence designated as the dependent variable. The results indicated that recurrence rate could be reduced by choosing double burr hole over single burr hole, and performing irrigation.
Hematoma volume and midline shift are factors that have a strong influence on patient outcome. Perel et al.18) described a prediction model for outcomes in patients with traumatic brain injury and reported that hematoma volume and midline shift were very important factors. Jacobs et al.7) reported that, in a study on 700 patients with traumatic brain injury, a greater degree of hematoma volume and midline shift was associated with poor outcome. In our study, the change in hematoma thickness and midline shift was statistically significantly higher in Group C; i.e., postoperative hematoma volume was distinctly reduced compared with the other two groups, and midline shift had effectively recovered. Therefore, the double BHD with saline irrigation may be more helpful in improving the outcome in patients with CSDH than the other two techniques.
The CSDH contains highly concentrated vaso-active cytokines, inflammatory mediators, and fibrinolytic factors.2728) Saito et al.24) described that since leaving these factors in high concentrations after surgery leads to an increase in recurrence of CSDH, complete evacuation of hematoma during surgery is a very important surgical goal. As mentioned above, our results indicate that double BHD with saline irrigation is the most effective technique for hematoma resolution compared to the other two techniques. Additionally, saline irrigation washes out residual cytokines and fibrinolytic factors; hence, it may be helpful in reducing postoperative recurrence. In summary, double BHD with saline irrigation may be the most effective technique for preventing the recurrence of CSDH.
Yasuda et al.31) performed a correlation analysis between coagulation abnormality and postoperative outcome in 160 patients with CSDH, and reported that the elevated INR resulted in increases in poor outcome and mortality index. In our study, coagulopathy was also found to be a statistically significant independent risk factor for recurrence of CSDH. Therefore, due consideration should be given to decide whether to operate on CSDH patients with elevated INR. Our findings may be helpful in making that decision.
Our study has the limitation of being a retrospective study. It may also be partially limited from having strict exclusion criteria for cases, which resulted in relatively small number of overall cases. Thus, a prospective and randomized control study with more cases is needed.
Our study compared three surgical techniques of BHD for CSDH at once. Among the three surgical techniques, double BHD with saline irrigation was found to be the most effective for hematoma resolution, recovery of midline shift, and reduction of postoperative recurrence rate. Therefore, in patients who are suspected of having a high risk of postoperative recurrence, selecting the double BHD with saline irrigation technique may be ideal. Moreover, we believe that our study will be helpful for future determination of an optimal surgical technique for CSDH.
References
1. Abouzari M, Rashidi A, Rezaii J, Esfandiari K, Asadollahi M, Aleali H, et al. The role of postoperative patient posture in the recurrence of traumatic chronic subdural hematoma after burr-hole surgery. Neurosurgery. 2007; 61:794–797. discussion 797PMID: 17986941.

2. Asano Y, Hasuo M, Takahashi I, Shimosawa S. [Recurrent cases of chronic subdural hematoma--its clinical review and serial CT findings]. No To Shinkei. 1992; 44:827–831. PMID: 1476812.
3. Cenic A, Bhandari M, Reddy K. Management of chronic subdural hematoma: a national survey and literature review. Can J Neurol Sci. 2005; 32:501–506. PMID: 16408582.

4. Chen JC, Levy ML. Causes, epidemiology, and risk factors of chronic subdural hematoma. Neurosurg Clin N Am. 2000; 11:399–406. PMID: 10918008.

5. Erol FS, Topsakal C, Faik Ozveren M, Kaplan M, Tiftikci MT. Irrigation vs. closed drainage in the treatment of chronic subdural hematoma. J Clin Neurosci. 2005; 12:261–263. PMID: 15851078.

6. Ishibashi A, Yokokura Y, Adachi H. A comparative study of treatments for chronic subdural hematoma: burr hole drainage versus burr hole drainage with irrigation. Kurume Med J. 2011; 58:35–39. PMID: 22027196.

7. Jacobs B, Beems T, van der Vliet TM, Diaz-Arrastia RR, Borm GF, Vos PE. Computed tomography and outcome in moderate and severe traumatic brain injury: hematoma volume and midline shift revisited. J Neurotrauma. 2011; 28:203–215. PMID: 21294647.

8. Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975; 1:480–484. PMID: 46957.
9. Jennett B, Snoek J, Bond MR, Brooks N. Disability after severe head injury: observations on the use of the Glasgow Outcome Scale. J Neurol Neurosurg Psychiatry. 1981; 44:285–293. PMID: 6453957.

10. Jeong SI, Kim SO, Won YS, Kwon YJ, Choi CS. Clinical Analysis of Risk Factors for Recurrence in Patients with Chronic Subdural Hematoma Undergoing Burr Hole Trephination. Korean J Neurotrauma. 2014; 10:15–21.

11. Kang MS, Koh HS, Kwon HJ, Choi SW, Kim SH, Youm JY. Factors Influencing Recurrent Chronic Subdural Hematoma after Surgery. J Korean Neurosurg Soc. 2007; 41:11–15.
12. Kansal R, Nadkarni T, Goel A. Single versus double burr hole drainage of chronic subdural hematomas. A study of 267 cases. J Clin Neurosci. 2010; 17:428–429. PMID: 20202850.

13. Krupp WF, Jans PJ. Treatment of chronic subdural haematoma with burr-hole craniostomy and closed drainage. Br J Neurosurg. 1995; 9:619–627. PMID: 8561934.

14. Lee JY, Ebel H, Ernestus RI, Klug N. Various surgical treatments of chronic subdural hematoma and outcome in 172 patients: is membranectomy necessary? Surg Neurol. 2004; 61:523–527. discussion 527-528PMID: 15165784.

15. Lind CR, Lind CJ, Mee EW. Reduction in the number of repeated operations for the treatment of subacute and chronic subdural hematomas by placement of subdural drains. J Neurosurg. 2003; 99:44–46. PMID: 12854742.

16. Mahapatra AK, Bansal S. Role of intracranial pressure monitoring in head injury: a prospective study. Neurol India. 1998; 46:109–114.
17. Markwalder TM, Seiler RW. Chronic subdural hematomas: to drain or not to drain? Neurosurgery. 1985; 16:185–188. PMID: 3974829.

18. MRC CRASH Trial Collaborators. Perel P, Arango M, Clayton T, Edwards P, Komolafe E, et al. Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients. BMJ. 2008; 336:425–429. PMID: 18270239.

19. Nakaguchi H, Tanishima T, Yoshimasu N. Factors in the natural history of chronic subdural hematomas that influence their postoperative recurrence. J Neurosurg. 2001; 95:256–262. PMID: 11780895.

20. Okada Y, Akai T, Okamoto K, Iida T, Takata H, Iizuka H. A comparative study of the treatment of chronic subdural hematoma--burr hole drainage versus burr hole irrigation. Surg Neurol. 2002; 57:405–409. discussion 410PMID: 12176202.

21. Pahatouridis D, Alexiou GA, Fotakopoulos G, Mihos E, Zigouris A, Drosos D, et al. Chronic subdural haematomas: a comparative study of an enlarged single burr hole versus double burr hole drainage. Neurosurg Rev. 2013; 36:151–154. discussion 154-155PMID: 22869256.

22. Regan JM, Worley E, Shelburne C, Pullarkat R, Watson JC. Burr hole washout versus craniotomy for chronic subdural hematoma: patient outcome and cost analysis. PLoS One. 2015; 10:e0115085. PMID: 25611468.

23. Robinson RG. Chronic subdural hematoma: surgical management in 133 patients. J Neurosurg. 1984; 61:263–268. PMID: 6737050.

24. Saito K, Ito H, Hasegawa T, Yamamoto S. Plasmin-alpha 2-plasmin inhibitor complex and alpha 2-plasmin inhibitor in chronic subdural hematoma. J Neurosurg. 1989; 70:68–72. PMID: 2521247.
25. Santarius T, Lawton R, Kirkpatrick PJ, Hutchinson PJ. The management of primary chronic subdural haematoma: a questionnaire survey of practice in the United Kingdom and the Republic of Ireland. Br J Neurosurg. 2008; 22:529–534. PMID: 18686063.

26. Smely C, Madlinger A, Scheremet R. Chronic subdural haematoma--a comparison of two different treatment modalities. Acta Neurochir (Wien). 1997; 139:818825–discussion 825-826. PMID: 9351986.
27. Suzuki K, Takano S, Nose T, Doi M, Ohashi N. Increased concentration of vascular endothelial growth factor (VEGF) in chronic subdural hematoma. J Trauma. 1999; 46:532–533. PMID: 10088869.
28. Taussky P, Fandino J, Landolt H. Number of burr holes as independent predictor of postoperative recurrence in chronic subdural haematoma. Br J Neurosurg. 2008; 22:279–282. PMID: 18348026.

29. Teasdale G, Jennett B. Assessment and prognosis of coma after head injury. Acta Neurochir (Wien). 1976; 34:45–55. PMID: 961490.

30. Weigel R, Schmiedek P, Krauss JK. Outcome of contemporary surgery for chronic subdural haematoma: evidence based review. J Neurol Neurosurg Psychiatry. 2003; 74:937–943. PMID: 12810784.

31. Yasuda CL, Morita ME, Nishimori FY, Yasuda AM, Alves HL. [Chronic subdural hematoma: study of 161 patients and the relationship with coagulation abnormalities]. Arq Neuropsiquiatr. 2003; 61:1011–1014. PMID: 14762608.
32. Zakaraia AM, Adnan JS, Haspani MS, Naing NN, Abdullah JM. Outcome of 2 different types of operative techniques practiced for chronic subdural hematoma in Malaysia: an analysis. Surg Neurol. 2008; 69:608–615. discussion 616PMID: 18486703.

FIGURE 1
The internal architectures of chronic subdural hematoma (CSDH). A: Axial view of non-enhance brain computed tomography shows a homogenous internal architecture of CSDH. B: Heterogeneous internal architecture.
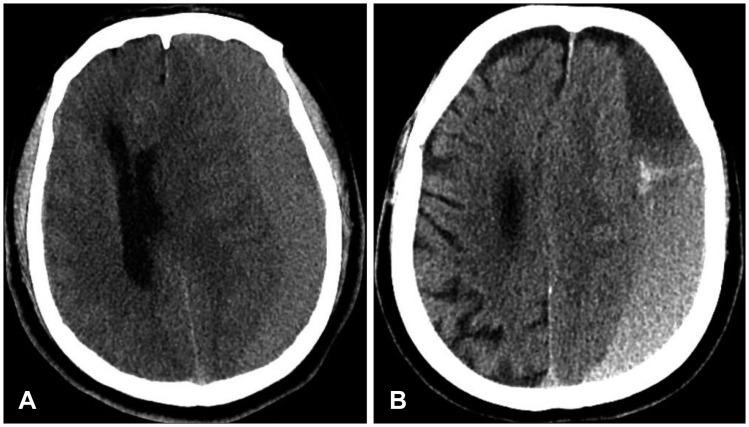
TABLE 1
Factors associated with the recurrence of chronic subdural hematoma analyzed by univariate logistic regression





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download