Abstract
Objective
The aim of our study was to classify the outer membrane of chronic subdural hematoma (CSDH) histologically and to determine the clinical and radiological meaning of the classified membranes.
Methods
The outer membrane specimen of 31 patients who underwent surgery for CSDH were acquired in this study. The specimen was classified into four types and each were analyzed of the symptoms on the admission day and during the period from trauma to surgery. The radiological features such as subdural fluid density, Hounsfield number, thickness of the hematoma, and midline shift were analyzed.
Results
There were 6% of type I, 29% of type II, 39% of type III, and 26% of type IV neomembranes. The cases of CSDH accompanied by neurologic deficit were highest from type IV of 63%, followed by type II with 56%. On the radiological findings such as Hounsfield unit, hematoma thickness and midline shift, only hematoma thickness between type II and III were statistically significant (p=0.021). The hematoma thickness and midline shift were greatest in type II. On computed tomography scans, the isodense, hyperdense and laminar type that shows the high recurrence rate formed 75% of type II and 67% of type IV while type III had the low possibility of recurrence rate (33%).
Although the pathophysiology of chronic subdural hematomas (CSDH) has not been clearly identified, there are many studies in progress, including the papers on the outer membrane's role on CSDH.37111718) Past studies were mainly on the electron microscopic findings of the outer membrane.1314) And these are not easy to apply in practice.
In one study, outer membrane was classified into 4 types pathologically according to maturity and intensity of the inflammatory reaction and hemorrhage.11) The study indicated that analyzing the clinical significance of each group of the outer membrane may be helpful to assume the prognosis of patients with CSDH.
We conducted this study in order to classify our specimen of the outer membrane of CSDH histologically and to review the clinical features according to the histological types of the outer membranes.
The outer membrane specimen of 31 patients who underwent surgery for CSDH between November 2004 and January 2013 were acquired through small craniotomy and the samples were cut to the size of 1×1 cm for the study. In the treatment of CSDH, we were able to remove organized hematomas or clots by irrigation much more easily and effectively with the small craniotomy (about 3 to 4 cm in diameter).9)
By using the paraffin blocks of the specimen, we executed hematoxylin and eosin staining and Elastica-van Gieson staining. Histological features of the outer membrane were classified into 4 types according to maturity and intensity of the inflammatory reaction and hemorrhage. Type I is non-inflammatory membrane, containing immature fibroblasts and collagen fibers. It was associated with very slight or sparse cell infiltration and neocapillaries. Type II is inflammatory membrane, consisting one layer of immature connective tissue. It was associated with marked cell infiltration and vascularization of the whole thickness. Type III is hemorrhagic-inflammatory membrane, consisted of two or three layers. It is consisted of large capillaries on the side of the dura mater and marked cell infiltration with many thin new vessels on the hematoma cavity. Type IV is scar-inflammatory membrane, which shows inflammatory cell infiltration, neovascularization and hemorrhage in the outer membrane of cicatrical tissue.11)
We analyzed the clinical and the radiologic findings of the patients applicable to each type. The clinical symptoms on the admission day were divided into two groups, one with the symptom of headache alone, and the other that accompanied neurologic deficit such as motor weakness, mental change and dysarthria. And the period from trauma to surgery was reviewed. The analyzed radiologic features include subdural fluid density, Hounsfield number, thickness of the hematoma, and the length of midline shift. The brain computed tomography (CT) findings of the subdural fluid density were classified by hyperdense, isodense, hypodense, and mixed density type; mixed density type was categorized into laminar, separated, gradation, and trabecular type.
To assess statistical significance, we used SPSS for windows version 19.0 (SPSS Inc., Chicago, IL, USA). Nonparametric data were compared using the Kruskal-Wallis and Mann-Whitney U-test. p-values <0.05 were considered significant.
Of the 31 patients, 24 were male with the average age of 61.2±15.2. Of those patients, one patient had a re-operation on account of recurrence in the same area after 8 months and two patients were operated the opposite side a few days after surgery due to increase the subdural fluid volume. Each of them was considered as a separate case. The cause of the head trauma of the patients are as follows; fourteen patients had the head trauma caused by falling while 10 didn't know the cause, 6 from traffic accident and 1 from the assault.
Histopathological analysis revealed 2 cases (6%) of type I, 9 cases (29%) of type II, 12 cases (39%) of type III, and 8 cases (26%) of type IV neomembranes (Figures 1, 2, 3, 4).
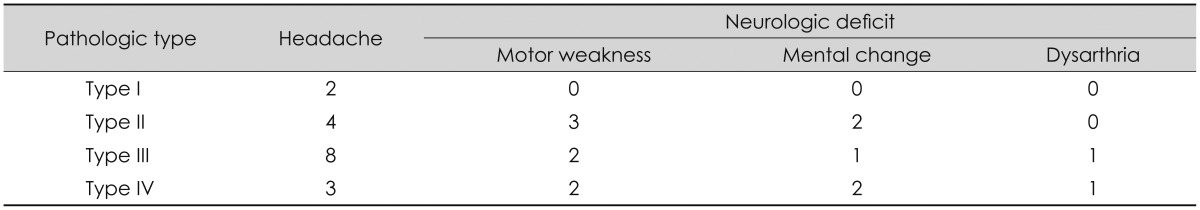
The cases of all 31 patients had the Glasgow Coma Scale (GCS) scores of more than 14, therefore there was no severe consciousness decline. In the clinical symptoms on the admission day, 17 cases reported simple headache, while 14 cases accompanied with neurological manifestation; 7 motor weakness, 5 mental changes and 2 dysarthria. The result of the analysis shows that according to the histopathological types of the outer membranes, neurologic deficit was accompanied highest with type IV by 63%, seconded by type II, 56%, followed by 33% of type III while type I didn't have any (Table 1).
The average period of trauma to surgery was 32.5±10.6 days in type I and 32.3±30.6 days, 36.3±40.1 days and 59.4±54.7 days in type II, III, IV respectively. And there was no statistical difference between each type (type I/II p=0.994, I/III p=0.779, I/IV p=0.345, II/III p=0.850, II/IV p=0.404, III/IV p=0.436).
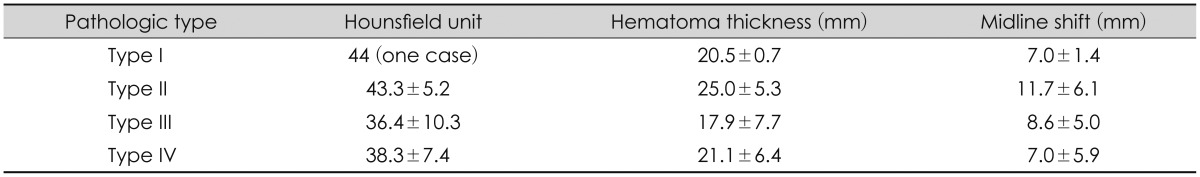
Out of 31 cases, 24 were examined by brain CT before the surgery while 7 used only the MRI. From the data of 24 brain CT performed before surgery, Hounsfield unit was 44 in type I (only one case), and type II, III, IV were 43.3±5.2, 36.4±10.3, 38.3±7.4 respectively. Immediate preoperative thickness of subdural hematoma was 20.5±0.7 mm in type I and 25.0±5.3 mm, 17.9±7.7 mm and 21.1±6.4 mm in type II, III, IV respectively. The midline shift of the preoperative image was 7.0±1.4 mm in type I and type II, III, IV was 11.7±6.1 mm, 8.6±5.0 mm, and 7.0±5.9 mm respectively (Table 2). On the radiological findings of the above, only hematoma thickness between type II and III were statistically significant (p=0.021).
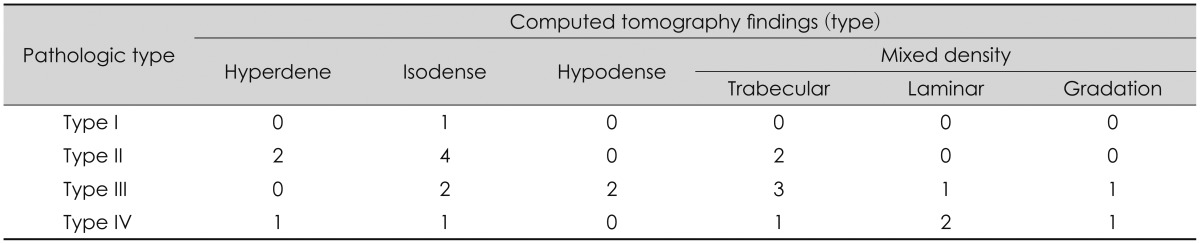
On CT scans, 11 patients (46%) had mixed density types, 8 patients (33%) had isodense types, 3 patients (13%) had hyperdense types, and 2 patients (8%) had hypodense types. The analysis through histopathological types of the outer membrane shows that type II had 2 hyperdense and 4 isodense type cases and type III and IV had a mixed density type high with 5 and 4 cases respectively. Of the mixed density, 6 cases were trabecular type, 3 were laminar type and 2 of gradation type (Table 3).
CSDH is prevalent among elderly populations worldwide, and its pathogenesis have been discussed in the literature for decades. With the increase of the senior population, the prevalence rate of the disease is also increasing. This chronic disease has several factors that affect the progression of the disease. Such interesting subject of study was particularly focused on the outer membrane of CSDH diversely, 3456711151718) and the neoformation of capillaries and vascular hyperpermeability of outer membrane found from CSDH is reported to be correlated with the progressive enlargement of CSDH.18) The outer neomembrane of CSDH contains multiple components, especially friable neovasculature, and local fibrinolytic factors, which may influence the formation of a hematoma.3) The newly formed blood vessels appear to be more permeable and fragile than pre-existing vessels.15)
Friede2) reported four pathologic type of membranous and membranous-hemorrhagic lesions in 46 necropsies. His classification was neomembrane (type I), hemorrhagic neomembrane (type II), hematoma (type III), and fibrosis (type IV). Nagahori et al.11) classified the histological features of the outer membrane into four types based on the maturity and intensity of the inflammatory reaction and hemorrhage. They pointed out, based on a comparison of the density of hematoma on CT and the histological findings of outer membranes, that exudation from inflammation is involved in the early stage of hematoma formation, and the inflammation and hemorrhage are involved in the subsequent stage of hematoma enlargement. According to the histological features and the period of time from trauma to surgery, they suggested that the outer membrane is considered to have changed from type I to II, III, and IV, in order.13) In our study, due to the wide deviation of the data although there was no statistical significant, however we were able to find a similar trend to the result of Nagahori et al.11)
Most of the acute subdural hematomas resolves into hypodensity on CT within 3 weeks. The density of the CSDH increases again slowly and continuously. The wider the surface area of outer membrane is, the more extensive the neovascularity becomes, and the higher the fibrinolytic activity within the hematoma is, the more blood will be mixed.10) The CT scan attenuation values and the size of the hematoma tended to increase with the maturity of the neomembranes.4) In other words, the density of the CSDH changes from isodensity to hypodensity into the mixed density as time passes.
We have confirmed through our research that the most of type I and II were isodense type while type III and IV were mixed density with the exception of 2 cases of hypodense type belonging under type III and 3 cases of hyperdense type, 2 coming from type II and one from type IV. The result points toward the fact that the outer membrane changes from type I to IV, assuring the result of the study done by Nagahori et al.11) However, a report contends that the mixed density of CSDH is resulted from multiple episodes of trauma, usually in the aged, making it hard to conclude that mixed density occur only from the rebleeding from neovascularization.
Another report shows that although histological studies of the outer membrane can account for the development of CSDH, the findings poorly correlate with clinical manifestations. A GCS score of 12 or less exclusively had type II neomembranes which may correlate clinically to the period of rapid expansion of a CSDH and subsequent decompensation. Type II neomembranes may represent an early stage of inflammatory membrane formation associated with a higher stage of membranous exudation into the hematoma cavity and therefore has a tendency to bleed.4)
In the radiological analysis such as Hounsfield unit, hematoma thickness and midline shift for each pathologic type, we had only one the statistical meaning from the hematoma thickness between type II and III alone. Looking at the overall trend of other results, Hounsfield unit show a gradual decrease in the type I, II, and III then a slight increase in type IV. The hematoma thickness and midline shift were greatest in type II. This result is consistent with the above paper which suggested type II membrane may be related to the rapid expansion of a CSDH.
As our study did not have the case of the significantly lower GCS score, we did the comparisons of the clinical features between the case only with the headache and the case accompanied by neurologic deficit. The result shows that type IV and II had a high number of cases accompanied by neurologic deficit, which is dissimilar with the report from above.
The appearance of hematoma on CT scans could be a predictor of the post-operative recurrence. In a clinical analysis of risk factors associated with the recurrence of CSDH, they suggest that the incidence of recurrence in the high- or mixed-density groups was significantly higher than those in the low- or iso-density groups.18) And a prospective study of 107 operated patients reported a different result in isodense group and further subdivided mixed group. They concluded that the high recurrence rate was reported in the isodense, hyperdense, laminar, and separated type, whereas the low recurrence was in the hypodense, gradation, and trabecular type.16) Based on the latter paper, the results of our experiments are as follows. The isodense, hyperdense and laminar type showed the high recurrence rate; 75% of type II and 67% of type IV while type III had the low possibility of recurrence rate (33%). Therefore, we suggested that type II and IV needed to be observed closely as the possibility of recurrence is higher on those types.
We classified the outer membrane of the CSDH in 4 types histologically and analyzed each type with clinical and radiological findings. Outer membrane was identified to have the tendency to develop from type I to IV in time and suggested that type II and type IV may have more risk on neurologic deficit and the high possibility of recurrence. In the future, the experiments will be necessary for inflammatory change and angiogenesis of the outer membrane of CSDH.
References
1. Ahn SY, Kim JH, Ha SK, Kim JH, Kwon TH, Park YK, et al. Clinical analysis of risk factors associated with the recurrence of chronic subdural hematoma. J Korean Neurotraumatol Soc. 2011; 7:68–73.

2. Friede RL. Incidence and distribution of neomembranes of dura mater. J Neurol Neurosurg Psychiatry. 1971; 34:439–446. PMID: 5096558.

3. Friede RL, Schachenmayr W. The origin ofsubdural neomembranes. II. Fine structural of neomembranes. Am J Pathol. 1978; 92:69–84. PMID: 686149.
4. Gandhoke GS, Kaif M, Choi L, Williamson RW, Nakaji P. Histopathological features of the outer membrane of chronic subdural hematoma and correlation with clinical and radiological features. J Clin Neurosci. 2013; 20:1398–1401. PMID: 23916760.

5. Hara M, Tamaki M, Aoyagi M, Ohno K. Possible role of cyclooxygenase-2 in developing chronic subdural hematoma. J Med Dent Sci. 2009; 56:101–106. PMID: 20099472.
6. Hong HJ, Kim YJ, Yi HJ, Ko Y, Oh SJ, Kim JM. Role of angiogenic growth factors and inflammatory cytokine on recurrence of chronic subdural hematoma. Surg Neurol. 2009; 71:161–165. discussion 165-166PMID: 18423527.

7. Ito H, Yamamoto S, Komai T, Mizukoshi H. Role of local hyperfibrinolysis in the etiology of chronic subdural hematoma. J Neurosurg. 1976; 45:26–31. PMID: 132513.

8. Jeong SI, Kim SO, Won YS, Kwon YJ, Choi CS. Clinical analysis of risk factors for recurrence in patients with chronic subdural hematoma undergoing burr hole trephination. Korean J Neurotrauma. 2014; 10:15–21.

9. Lee JK, Choi JH, Kim CH, Lee HK, Moon JG. Chronic subdural hematomas: a comparative study of three types of operative procedures. J Korean Neurosurg Soc. 2009; 46:210–214. PMID: 19844620.
10. Lee KS, Bae WK, Bae HG, Doh JW, Yun IG. The computed tomographic attenuation and the age of subdural hematomas. J Korean Med Sci. 1997; 12:353–359. PMID: 9288636.

11. Nagahori T, Nishijima M, Takaku A. [Histological study of the outer membrane of chronic subdural hematoma: possible mechanism for expansion of hematoma cavity]. No Shinkei Geka. 1993; 21:697–701. PMID: 8361567.
12. Park HR, Lee KS, Shim JJ, Yoon SM, Bae HG, Doh JW. Multiple densities of the chronic subdural hematoma in CT scans. J Korean Neurosurg Soc. 2013; 54:38–41. PMID: 24044079.

13. Sato S, Suzuki J. Ultrastructural observations of the capsule of chronic subdural hematoma in various clinical stages. J Neurosurg. 1975; 43:569–578. PMID: 1181389.

14. Schachenmayr W, Friede RL. The origin of subdural neomembranes. I. Fine structure of the dura-arachnoid interface in man. Am J Pathol. 1978; 92:53–68. PMID: 686148.
15. Shono T, Inamura T, Morioka T, Matsumoto K, Suzuki SO, Ikezaki K, et al. Vascular endothelial growth factor in chronic subdural haematomas. J Clin Neurosci. 2001; 8:411–415. PMID: 11535006.

16. Stanišić M, Hald J, Rasmussen IA, Pripp AH, Ivanović J, Kolstad F, et al. Volume and densities of chronic subdural haematoma obtained from CT imaging as predictors of postoperative recurrence: a prospective study of 107 operated patients. Acta Neurochir (Wien). 2013; 155:323–333. discussion 333PMID: 23229873.

17. Suzuki K, Takano S, Nose T, Doi M, Ohashi N. Increased concentration of vascular endothelial growth factor (VEGF) in chronic subdural hematoma. J Trauma. 1999; 46:532–533. PMID: 10088869.
18. Vaquero J, Zurita M, Cincu R. Vascular endothelial growth-permeability factor in granulation tissue of chronic subdural haematomas. Acta Neurochir (Wien). 2002; 144:343–346. discussion 347PMID: 12021880.
FIGURE 1
Photomicrograph of type I non-inflammatory membrane stained with hematoxylin and eosin and Elastica-van Gieson staining under light microscopy (original magnification, ×100).
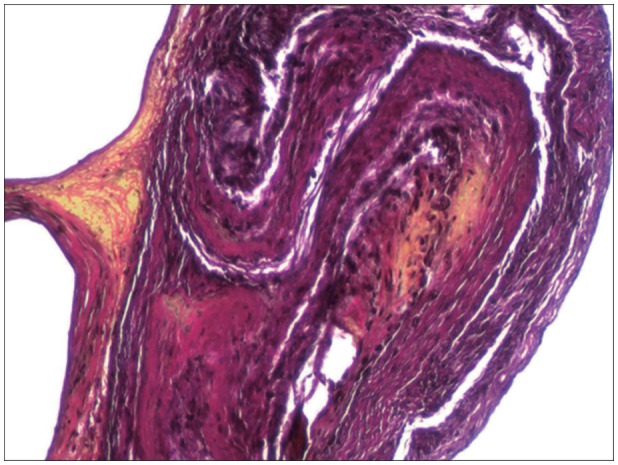
FIGURE 2
Photomicrograph of type II inflammatory membrane stained with hematoxylin and eosin and Elastica-van Gieson staining under light microscopy (original magnification, ×100).
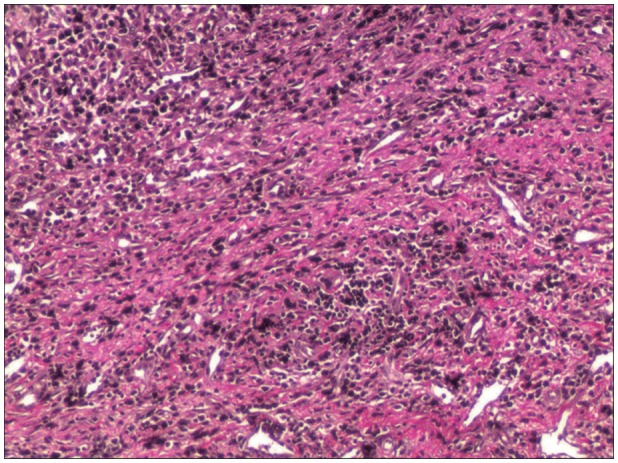
FIGURE 3
Photomicrograph of type III hemorrhagic inflammatory membrane stained with hematoxylin and eosin and Elastica-van Gieson staining under light microscopy (original magnification, ×100).
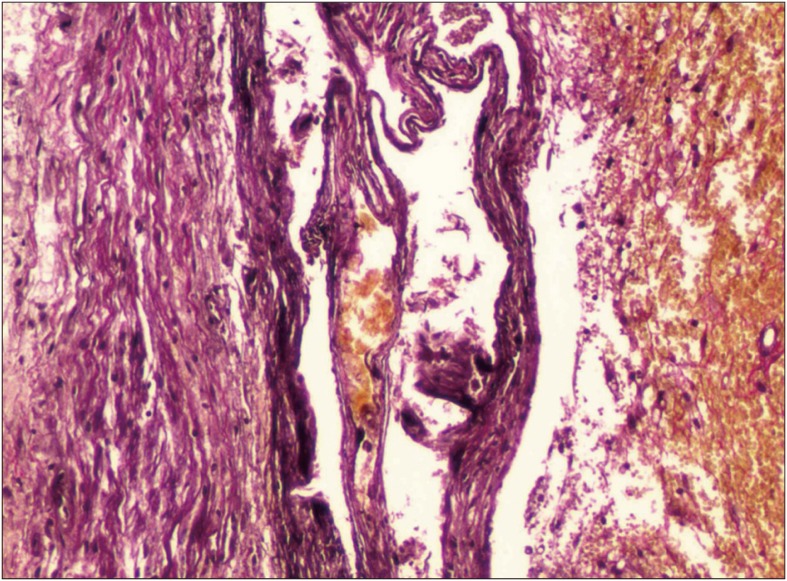
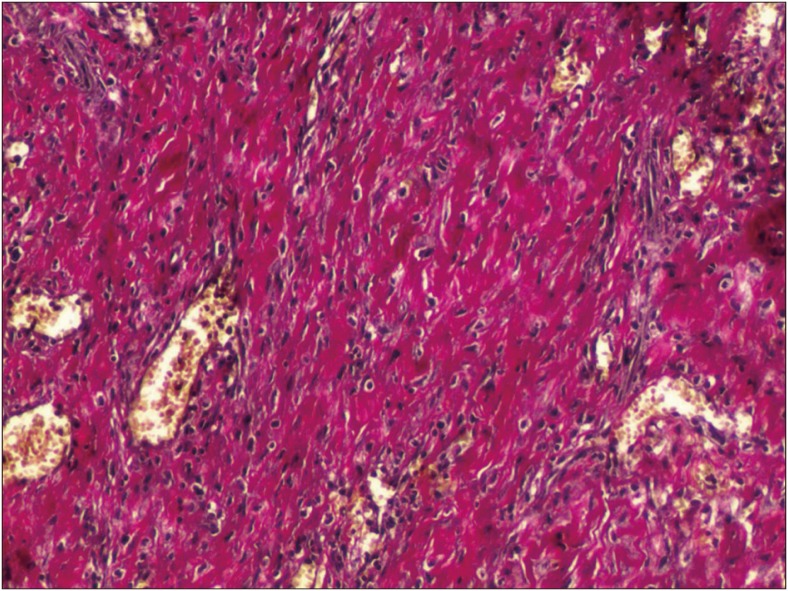
FIGURE 4
Photomicrograph of type IV scar inflammatory membrane stained with hematoxylin and eosin and Elastica-van Gieson staining under light microscopy (original magnification, ×100).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download