Abstract
Objective
Chronic subdural hematoma (CSDH) is a common form of extra axial hemorrhage in the elderly. A surgical procedures such as a burr hole trephination are used for the CSDH treatment. The recurrence rate of CSDH is reported to range from 2.3 to 33%. In the current study, we focused on the determination of risk factors associated with the recurrence of CSDH.
Methods
We retrospectively reviewed 368 consecutive patients with CSDH treated by burr hole trephination. Univariate and multivariate analysis were performed to describe the relationships between clinical and radiological factors as well as the recurrence of CSDH.
Results
Totally 31 (8.4%) patients experienced a recurrence of CSDH in our study. The male group (10.2%) had a higher recurrence rate than the female group (3.1%). Also patients with malignant neoplasm history showed a high recurrence rate (17.9%). The recurrence rate of single layer CSDH (13.1%) and isodensity CSDH (11.7%) was highly significant also.
Go to : 
A chronic subdural hematoma (CSDH) is a well-known disease of intracranial hemorrhage in old people. The burr hole trephination is the usual treatment of CSDH with a good outcome and prognosis.
Despite the simple treatment of CSDH, the recurrence rate of CSDH is not low. The recurrence rate of CSDH ranged between 10% and 33% in previous studies.26) Many studies have been reported on factors associated with the recurrence of CSDH; however, the results were inconsistent.
The purpose of this study was to identify risk factors for the recurrence of CSDH. We hypothesized that some factors including age, sex, underlying disease, radiological images and surgical procedures affect the recurrence of CSDH. Series of 368 surgical patients were retrospectively evaluated and analyzed to support this hypothesis.
Go to : 
We retrospectively evaluated 368 patients who underwent a surgery with CSDH. The patients visited the department of neurosurgery at Seoul National University Bundang Hospital between January 1993 and December 2013. This study was approved by the Institutional Review Board at Seoul National University Bundang Hospital (B-1405/250-103). The study population included 272 men and 96 women with a mean age of 68.61 years (range 18 to 99 years). Recurrent CSDH was defined as an increase in the hemorrhage volume in the identical subdural space following a previous burr hole trephination.24) Computed tomography (CT) was used to diagnose all cases of CSDH. All patients with CSDH following another neurosurgical surgery were excluded. The patients were divided into two groups: the non-recurrent group and the recurrent group.
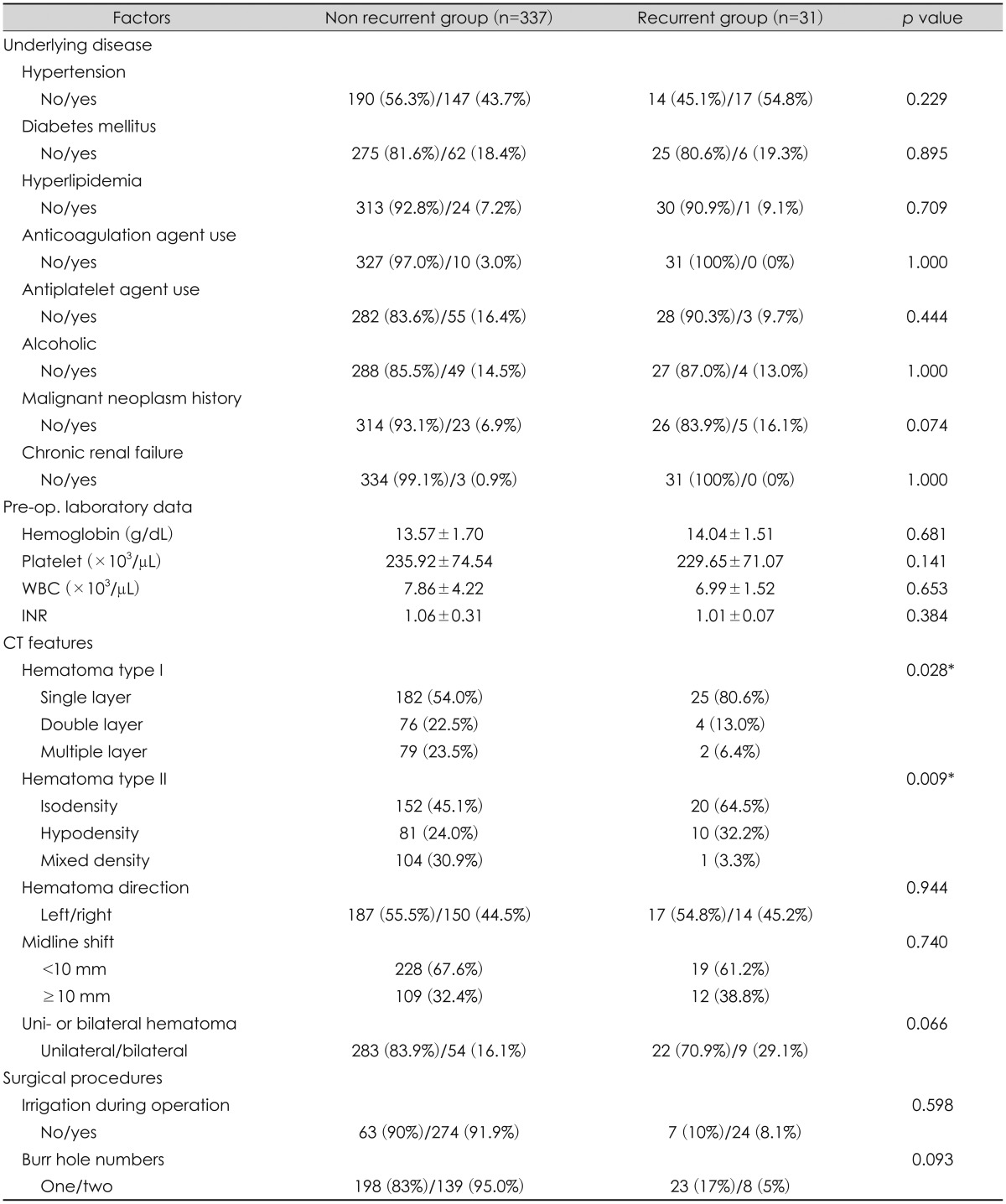
Patients' medical histories were assessed and preoperative underlying diseases were recorded. Age, sex, hypertension, diabetes mellitus (DM) and hyperlipidemia, chronic renal failure, malignant neoplasm history and alcohol consumption were evaluated. Patients were considered to have hypertension if systolic blood pressure (BP) >160 mm Hg or diastolic BP >95 mm Hg or if patients used antihypertensive drug. Person who used DM drugs or has initial blood sugar level >200 was considered as DM patients. Hyperlipidemia was defined as abnormally elevated levels of any or all lipids or lipoprotein in the blood. Person who used anti hyperlipidemia drugs was included. Excessive chronic alcohol consumption was defined as 3 days/week for 6 months. Malignant neoplasm history was considered if the patients have a history of malignant neoplasm before diagnosis of CSDH.
Also preoperative laboratory findings and the use of antiplatelet as well as anticoagulation drugs were collected from the medical chart. Furthermore, we reviewed the presence of head trauma history, initial symptoms, time interval between the 1st operation and the 2nd operation and the surgical procedures.
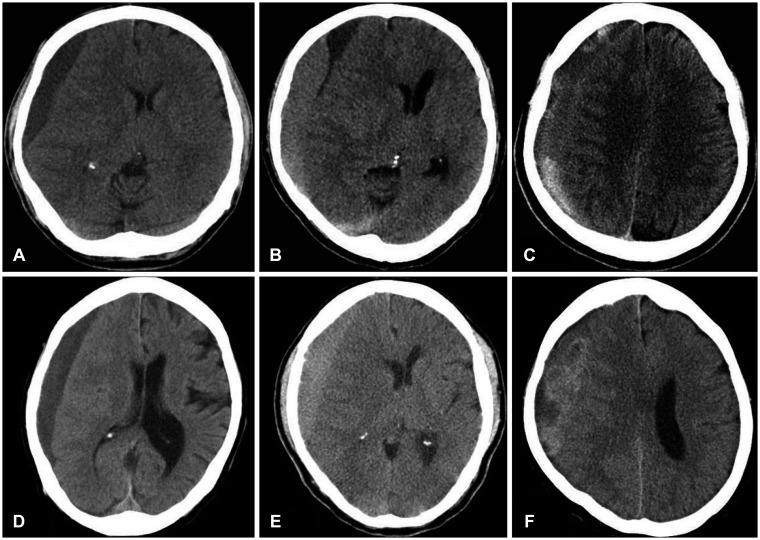
Characteristics of a hematoma were mainly assessed by CT (Figure 1). It was categorized as a single, double or as multiple layers according to the layers degree. A single layer hematoma was defined as one layer covering over brain cortex. A double layers hematoma was defined as two layers covering over brain cortex. A multiple layers hematoma was defined as above 3 layers covering the brain cortex. Additionally, characteristics of the hematoma were categorized as isodensity, hypodensity and mixed density according to the hematoma density.9) The hyperdensity hematoma was excluded from this study, because it is an acute subdural hematoma. The degree of the midline shift, the hematoma thickness and hematoma site were measured also. The midline shift was measured as the distance from the septum pellucidum point between the anterior horns of the lateral ventricles to a perpendicular line connecting the anterior and posterior insertions of the falx cerebri.22) The hematoma thickness was defined as the maximal thickness of the hematoma on the upper part level of the lateral ventricle. The hematoma site was measured also. Those radiological measurements were evaluated by a single investigator.
A standard surgical procedure was performed for all CSDH patients. The CSDH was removed by burr hole trephination under local anesthesia. After a dural incision, the outer membrane of the CSDH was opened and a silicon catheter was inserted to the hematoma cavity. Irrigation with isotonic saline solution was done in some patients by a silicon catheter. An external continuous drainage system was maintained for 2 to 3 days. We recommend the patients to lock the closed system drainage during walking or moving to prevent a possible over-drainage. The drainage tube was removed after the CT scan.
SPSS software (version 19.0, SPSS Inc., Chicago, IL, USA) was used for data analysis. We identified variables associated with the recurrence of CSDH with univariate and multivariate statistical analysis. Independent sample t-test and chi-squared test (or Fisher exact test) were used for univariate statistical analysis. In addition, a logistic regression model was used for multivariate statistical analysis. The relation of risk factors to CSDH recurrence was expressed as a 95% confidence interval and an odds ratio. Statistical significance was considered if the p-value was <0.05.
Go to : 
We reviewed 368 patients with CSDH: 272 males (73.9%) and 96 females (26.1%) with a mean age of 68.6 (±13.12) years. Table 1 shows demographics and the clinical presentation of CSDH patients. The total CSDH recurrence rate was 8.4% (31 out of 368). The male group showed a 10.2% (28 out of 272) recurrence rate, while the female group showed a 3.1% (3 out of 96) recurrence rate. The difference was statistically significant (p=0.032) (Table 1).
Only 169 patients (45.9%) had a history of trauma; 158 (46.9%) patients in the non-recurrent group and 11 (35.5%) patients in the recurrent group (p=0.233). However, 199 patients (54.1%) did not have an obvious trauma history.
Headache was the most common presentation in our CSDH patients; 61.5% (207 out of 337) in the non-recurrent group and 64.5% (20 out of 31) in the recurrent group. Other symptoms contained motor weakness, dysarthria and seizure, nausea and vomiting and mental change. The presentations were not significant different between the recurrent and the non-recurrent group.
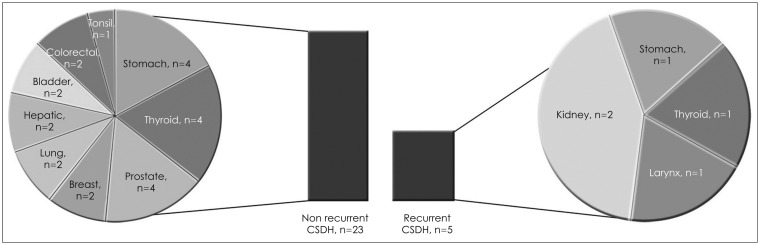
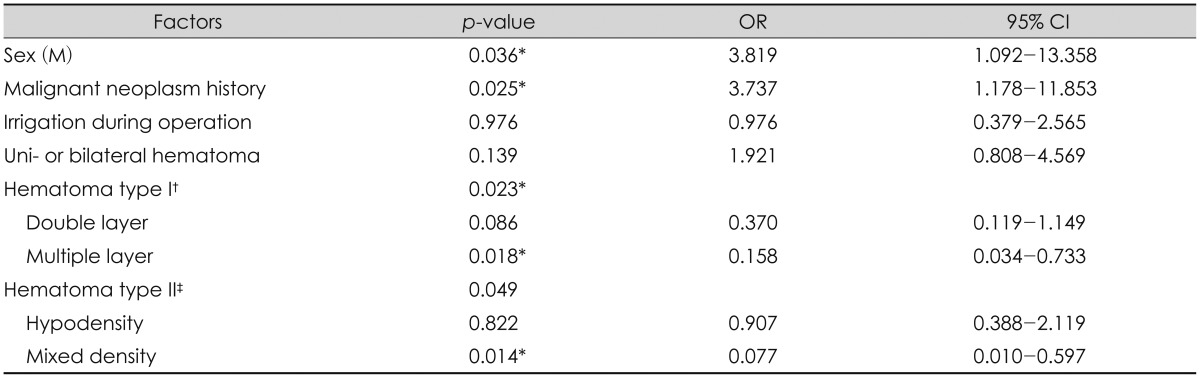
Hypertension, DM and hyperlipidemia, chronic renal failure, malignant neoplasm history and alcohol consumption were analyzed by a single investigator. Preoperative laboratory findings and the use of antiplatelet as well as anticoagulation drugs were also analyzed. Table 2 shows the underlying diseases and the laboratory data of the CSDH patients. All variables were not significant in the CSDH recurrence except of a malignant neoplasm history. Interestingly, total 28 patients (28 out of 368) demonstrated a history of malignant neoplasm; 5 patients in the recurrent CSDH group and 23 patients in the non-recurrent CSDH group. No significant difference was seen in the univariate analysis (p=0.074). However, it was significantly different in multivariate logistic analysis (p=0.025) (Table 3). Various malignant neoplasm history in CSDH patients are described in Figure 2.
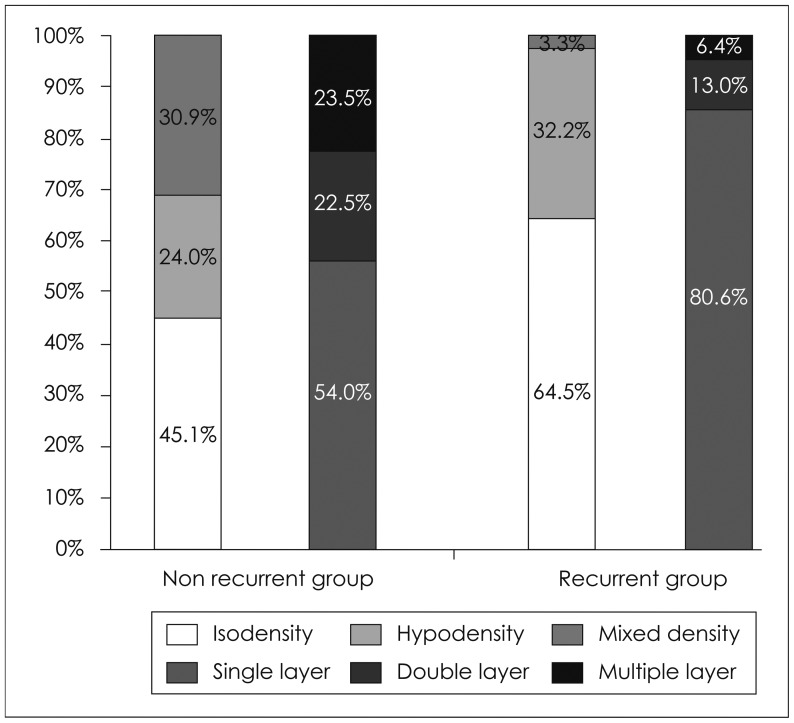
CT findings are summarized in Table 2. In the univariate analysis was the recurrence of CSDH not significantly associated with preoperative midline shift (p=0.740), hematoma site (p=0.944) and maximal width (p=0.18) of CSDH. However, the recurrence rate of CSDH was significantly related with the preoperative hematoma type. Hematoma layers significantly affected the recurrence of CSDH based on the CT. A single layer hematoma showed a CSDH recurrence rate of 80.6% (25 out of 31) whereas a double layer showed 13.0% (4 out of 31) and a multiple layer 1.1% (2 out of 31) (p=0.028). The hematoma density was also significantly related to the recurrence of CSDH. The isodensity recurrence of CSDH showed was 64.5% (20 out of 31) whereas the hypodensity recurrence of CSDH showed 32.2% (10 out of 31) and the mixed density recurrence rate of CSDH was 3.3% (1 out of 31) (p=0.009).
Hematoma characteristics are listed schematically in Figure 3. Isodensity (64.5%) and single layer (80%) rates in recurrent group was higher (45.1% and 54.0%) than those rates in non-recurrent group. Multivariate logistic analysis also shows hematoma types were also meaningful in recurrence rates (Table 3).
Factors associated to our surgical procedures are summarized in Table 2. The number of burr holes was not related to the recurrence of CSDH in univariate analysis (p=0.093) and in logistic multivariate analysis (p=0.066). Irrigation was also not significantly meaningful to the recurrence of CSDH in univariate analysis (p=0.598) and in logistic multivariate analysis (p=0.97). Our average recurrence interval was 38.26 (±4.05) days. Also a 2nd operation was performed through previous a burr hole site or a new burr hole site.
Almost all of our patients had great postoperative outcomes. Although 4 patients (4 of 368) needed a 2nd burr hole trephination, the overall outcome of 364 patients (364 of 368) was good. Only 4 patients needed an additional craniotomy and hematoma removal, because CSDH developed an acute subdural hematoma after the burr hole trephination.
Go to : 
Many researchers reported risk factors related to the recurrence of CSDH including age, alcohol abuse, bilateral CSDH, hematoma density and technical surgical procedures.214242527) However, risk factors were generally inconsistent. Therefore, we assessed the risk factors associated with the recurrence of CSDH through univariate and multivariate analysis. Thereby, we came to the conclusion that sex, malignant neoplasm history and the hematoma type on the CT scan were related to the recurrence of CSDH.
Generally, an occurrence of CSDH in older people comes from an age-related decrease in brain volume, increase of the tension on the cerebral bridging vein and an increased venous fragility associated with aging.321) This tendency might be different in the male and the female group. The male gender was definitely related to the recurrence of CSDH with a significant difference between both groups (p=0.032) (Table 1). Generally, male are predisposed to develop CSDH.3) Our study also showed trauma history of the male was much than those of the female consistently. In fact, the male group had a much higher chance to suffer from a trauma to the head than the female group. There is a theoretic possibility that higher levels of estrogen in the female group may have some protective effect on capillaries.21) In addition, estrogen is related to the repair of damaged vessels, because estrogen is known to induce vascular repair and angiogenesis.4)
Many studies revealed an apparent head trauma history up to 74.4% of CSDH.3624) However, our data showed a definite trauma history in only 169 patients (45.9%) (169 out of 368). If a minor trauma is so trivial, people tend to forget their trauma history. Furthermore, indirect trauma is also reported as important factor. Although 50% of the patients had a history of fall, patients did not hit their head on the ground.18) Even if it is not absolutely proportional to the occurrence of CSDH, the head trauma history is very important in CSDH.
Interestingly was the malignant neoplasm history statistically significant in our study. It was insignificant in univariate analysis (p=0.074), but significant in multivariate analysis (p=0.025). Patients with a malignant neoplasm history showed a higher CSDH recurrence rate (16.1%) (5 out of 31) than other patients (6.9%) (23 out of 337).
The frequency of intracerebral hemorrhage in malignant neoplasm has been reported different and varied depending on the underlying malignancy.1520) Risk factors for intracerebral hemorrhage include neovascularization, vascular invasion and coagulation imbalance.1217) Also, many case reports showed a relationship between brain metastasis of malignant neoplasm and CSDH.181016) Authors suggested a multifactorial pathogenesis of CSDH in malignancy such as dural vessel occlusion and rupture by tumor cells, tumor necrosis and chemotherapy-induced thrombocytopenia or disseminated intravascular coagulation secondary to an underlying malignancy.11) Authors suggested that CSDH seems easily to occur in patients with malignant neoplasm. However, our study could not show a definite metastatic brain tumor in any CSDH patient. Because our sample size was small, it is not correct that previous malignant neoplasm can affect recurrence of CSDH exactly. Further research is additionally needed to look for a relationship between CSDH recurrence and a malignant neoplasm history.
A CT is well known as a useful tool for the diagnosis of CSDH. Therefore, we mainly used CT to evaluate CSDH. Our data showed single layer hematoma was significant in the recurrence of CSDH. Single layer hematoma showed 80.6% (25 out of 31) recurrence rates of CSDH.
The recurrence rate of CSDH showing isodensity was 64.5% (20 out of 31) (p=0.009). These findings correspond well to one result reported in the literature.22) Therefore, the isodensity of CSDH might also be important as a radiological factor of CSDH recurrence. However, Mori and Maeda13) and Tsai et al.25) could not detect a positive correlation between radiological images based on CT density and recurrence. Saito et al.19) also did not detect a positive association between radiological images and CSDH recurrence. He concluded that chemical factors of the hematoma membrane may show a better relationship with the CSDH recurrence than radiological characteristics. His study showed only for chemical factors such as vascular endothelial growth factor and transforming growth factor-β influence on the vascularization of the hematoma membrane. Further studies are consistently required as the relationship between radiological factors and CSDH recurrence remains still unclear.
We mostly performed burr hole trephination through parietal eminence. The number of burr holes in each operation showed no specific differences in our study (p=0.093). Other studies showed no significant differences in the recurrence rate between 1 hole and 2 holes trephination also.57) However, Taussky et al.23) found a significantly higher rate of recurrence for 1 hole trephination. Their data showed a longer length of hospital stay and an increased rate of wound infection in the group with 1 hole trephination.
Go to : 
Some factors have been shown as relatively significant. In our study were sex, history of a malignant neoplasm and the type of hematoma (single and isodensity) factors related to the recurrence of CSDH. Therefore, CSDH patients with risk factors should be followed closely, even if there is no trauma history present.
Go to : 
Notes
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI10C2020).
Go to : 
References
1. Abdulhamid MM, Li YM, Hall WA. Spontaneous acute subdural hematoma as the initial manifestation of chronic myeloid leukemia. J Neurooncol. 2011; 101:513–516. PMID: 20582615.

2. Abouzari M, Rashidi A, Rezaii J, Esfandiari K, Asadollahi M, Aleali H, et al. The role of postoperative patient posture in the recurrence of traumatic chronic subdural hematoma after burr-hole surgery. Neurosurgery. 2007; 61:794–797. discussion 797PMID: 17986941.

3. Adhiyaman V, Asghar M, Ganeshram KN, Bhowmick BK. Chronic subdural haematoma in the elderly. Postgrad Med J. 2002; 78:71–75. PMID: 11807186.
4. Barnabas O, Wang H, Gao XM. Role of estrogen in angiogenesis in cardiovascular diseases. J Geriatr Cardiol. 2013; 10:377–382. PMID: 24454332.
5. Han HJ, Park CW, Kim EY, Yoo CJ, Kim YB, Kim WK. One vs. Two Burr Hole Craniostomy in Surgical Treatment of Chronic Subdural Hematoma. J Korean Neurosurg Soc. 2009; 46:87–92. PMID: 19763208.

6. Huang YH, Yang KY, Lee TC, Liao CC. Bilateral chronic subdural hematoma: what is the clinical significance? Int J Surg. 2013; 11:544–548. PMID: 23707986.

7. Kansal R, Nadkarni T, Goel A. Single versus double burr hole drainage of chronic subdural hematomas. A study of 267 cases. J Clin Neurosci. 2010; 17:428–429. PMID: 20202850.

8. Kimura S, Kotani A, Takimoto T, Yoshino A, Katayama Y. Acute aggravation of subdural fluid collection associated with dural metastasis of malignant neoplasms: case report and review of the literature. Brain Tumor Pathol. 2014; 31:299–303. PMID: 24036578.

9. Kostanian V, Choi JC, Liker MA, Go JL, Zee CS. Computed tomographic characteristics of chronic subdural hematomas. Neurosurg Clin N Am. 2000; 11:479–489. PMID: 10918018.

10. Kuan-Yin T, Dueng-Yuan H, Hsin-I M. Subdural hematoma associated with skull and dural metastasis of gastric carcinoma: a case report. Turk Neurosurg. 2013; 23:796–799. PMID: 24310465.

11. Laigle-Donadey F, Taillibert S, Mokhtari K, Hildebrand J, Delattre JY. Dural metastases. J Neurooncol. 2005; 75:57–61. PMID: 16215816.

12. Liwnicz BH, Wu SZ, Tew JM Jr. The relationship between the capillary structure and hemorrhage in gliomas. J Neurosurg. 1987; 66:536–541. PMID: 3031239.

13. Mori K, Maeda M. Surgical treatment of chronic subdural hematoma in 500 consecutive cases: clinical characteristics, surgical outcome, complications, and recurrence rate. Neurol Med Chir (Tokyo). 2001; 41:371–381. PMID: 11561347.

14. Nagatani K, Takeuchi S, Sakakibara F, Otani N, Nawashiro H. Radiological factors related to recurrence of chronic subdural hematoma. Acta Neurochir (Wien). 2011; 153:1713. PMID: 21347577.

15. Navi BB, Reichman JS, Berlin D, Reiner AS, Panageas KS, Segal AZ, et al. Intracerebral and subarachnoid hemorrhage in patients with cancer. Neurology. 2010; 74:494–501. PMID: 20142616.

16. Prasad BC, Chandra VV, Varaprasad G. Dural metastases in chronic myeloid leukemia presenting as subdural hematoma. Turk Neurosurg. 2012; 22:777–778. PMID: 23208914.

17. Rogers LR. Cerebrovascular complications in cancer patients. Neurol Clin. 2003; 21:167–192. PMID: 12690649.

18. Rozzelle CJ, Wofford JL, Branch CL. Predictors of hospital mortality in older patients with subdural hematoma. J Am Geriatr Soc. 1995; 43:240–244. PMID: 7884110.

19. Saito A, Narisawa A, Takasawa H, Morita T, Sannohe S, Sasaki T, et al. Expression of the TGF-β-ALK-1 pathway in dura and the outer membrane of chronic subdural hematomas. Neurol Med Chir (Tokyo). 2014; 54:357–362. PMID: 24305026.

20. Schrader B, Barth H, Lang EW, Buhl R, Hugo HH, Biederer J, et al. Spontaneous intracranial haematomas caused by neoplasms. Acta Neurochir (Wien). 2000; 142:979–985. PMID: 11086806.

21. Spallone A, Giuffrè R, Gagliardi FM, Vagnozzi R. Chronic subdural hematoma in extremely aged patients. Eur Neurol. 1989; 29:18–22. PMID: 2707288.

22. Stanišić M, Hald J, Rasmussen IA, Pripp AH, Ivanović J, Kolstad F, et al. Volume and densities of chronic subdural haematoma obtained from CT imaging as predictors of postoperative recurrence: a prospective study of 107 operated patients. Acta Neurochir (Wien). 2013; 155:323–333. discussion 333PMID: 23229873.

23. Taussky P, Fandino J, Landolt H. Number of burr holes as independent predictor of postoperative recurrence in chronic subdural haematoma. Br J Neurosurg. 2008; 22:279–282. PMID: 18348026.

24. Torihashi K, Sadamasa N, Yoshida K, Narumi O, Chin M, Yamagata S. Independent predictors for recurrence of chronic subdural hematoma: a review of 343 consecutive surgical cases. Neurosurgery. 2008; 63:1125–1129. discussion 1129PMID: 19008766.
25. Tsai TH, Lieu AS, Hwang SL, Huang TY, Hwang YF. A comparative study of the patients with bilateral or unilateral chronic subdural hematoma: precipitating factors and postoperative outcomes. J Trauma. 2010; 68:571–575. PMID: 20065879.

26. Weigel R, Schmiedek P, Krauss JK. Outcome of contemporary surgery for chronic subdural haematoma: evidence based review. J Neurol Neurosurg Psychiatry. 2003; 74:937–943. PMID: 12810784.

27. Yamamoto H, Hirashima Y, Hamada H, Hayashi N, Origasa H, Endo S. Independent predictors of recurrence of chronic subdural hematoma: results of multivariate analysis performed using a logistic regression model. J Neurosurg. 2003; 98:1217–1221. PMID: 12816267.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download