This article has been
cited by other articles in ScienceCentral.
Abstract
Objective
After injury to the central nervous system (CNS), glial scar tissue is formed in the process of wound healing. This can be is a clinical problem because it interferes with axonal regeneration and functional recovery. It is known that intracellular proteins, including the glial fibrillary acidic protein (GFAP), nestin, and vimentin increase in the astrocytes after an injury to the CNS. By studying the time course and co-expression pattern of these intracellular proteins, this study will attempt to prove that these proteins are involved in the processes of glial scar formation.
Methods
Twenty-five male Sprague-Dawley rats were used in this study. Bregma of the cerebral cortex, an area was incised with a sharp blade, and perfusion was performed. The expressions of the intracellular proteins were assayed, while the co-localization of the intermediate filament (GFAP, nestin, and vimentin) and A2B5 were examined.
Results
At 12 hours, the GFAP was expressed in the white matter underlying the lesion, and in the cerebral cortex. Nestin was expressed in the astrocytes in the perilesional area after 3 days, while A2B5 was observed in the edge of the wound at 12 hours post-injury, with its expression reaching a peak at 7 days. Vimentin was detected in the white matter at 12 hours, and in the cortex, reaching a peak at 7 days.
Conclusion
In the processes of glial scar formation, nestin, vimentin, and A2B5 were revealed in the astrocytes, and these factors may be involved in the division, proliferation, and transportation of the astrocytes.
Go to :

Keywords: Astrocyte, Glial fibrillary acidic protein, Nestin, Vimentin, Gliosis
Introduction
Repair after an injury to the central nervous system (CNS) results in a series of cellular processes, involving astrocytes, oligodendrocytes, and microglia. This eventually leads to the formation of a scar, which is a clinical problem because it interferes with axonal regeneration.
4) In order to improve brain recovery after an injury, it is necessary to block an important step in the process of scar formation. However, the exact mechanism of scar formation is not yet known.
47141519) One of the most distinct morphological changes in the process of glial scar formation is the increased expression of the glial fibrillary acidic protein (GFAP) in the cells surrounding the injury site.
3)
By studying the time course and expression pattern of the GFAP, and several markers of cell proliferation, differentiation, and migration following injury to the brain, we aim to show that the activation of the astrocytes contributes to the scar formation after brain injury.
Go to :

Materials and Methods
Twenty-five male Sprague-Dawley rats (180-250 g) were used in this study, including 24 rats with cortical cuts and two controls. For the surgery, the rats were anesthetized with ketamine (50 mg/kg) and xylazine (8 mg/kg), and placed in a prone position in a stereotaxic frame. The body temperature was monitored and maintained using a heating pad. The scalp was incised along the midline, and the skull was drilled on both sides, 2 mm posterior to the bregma and 5 mm lateral to the midline. After opening the dura, a 3 to 4 mm long and 3 mm deep cortical cut was made with a steel micro blade, making an effort to spare the cortical blood vessels. The scalp was sutured with 4.0 silk, and the rats were allowed to survive for 12 hours and 1, 3, 7, or 14 days.
For fixation, the rats were anesthetized with sodium pentobarbital (60 mg/kg), and perfused intracardially with 100 mL of normal saline containing 500 units of heparin sodium, followed by 700 mL of freshly depolymerized 4% paraformaldehyde in a phosphate buffer (PB; 0.05 M, pH 7.2). The brains were removed, post-fixed in the fixative used for perfusion for 3 hours, and stored in cold PB. Blocks containing the sites of the cortical injury were cut using a vibratome at 50 µm, and stored in PB at 4℃.
Immunohistochemistry required the sections to be pretreated with 50% ethanol in phosphate-buffered saline (PBS; 0.05 M, pH 7.2) for 30 minutes, in order to improve antibody penetration. Additionally, the sections were pretreated with 10% normal donkey serum in PBS, to mask nonspecific secondary antibody binding sites, and then incubated in a mixture of two primary antibodies. We combined our rabbit anti-GFAP antibody (1:150) with either mouse anti-nestin (1:200), anti-vimentin (1:200), or anti-A2B5 (1:100) antibodies in PBS overnight, at room temperature. After several rinses and incubation in 2 (1:200; Vector). After several rinses, the sections were mounted on subbed slides, coverslipped with VECTASHIELD (Vector Laboratories, Burlingame, CA, USA), and examined using a confocal microscope. The confocal images were collected in the TIFF format, while the contrast and brightness were adjusted using Photoshop (Adobe Systems, San Jose, CA, USA).
For quantification, the degrees of expression of all five markers in the cells of the gray and white matter, within 500 µm of the edges of the wound, were scored on a 4-point scale (from "-" to "+++") in 3-5 confocal images, per double staining, per time point. To eliminate observer bias, the sets of the images were scored independently by two or three investigators blind to the source image; the variation between the scores assigned by the different investigators was analyzed using the ANOVA (Microsoft Excel, Microsoft, Redmond, WA, USA).
Go to :

Results
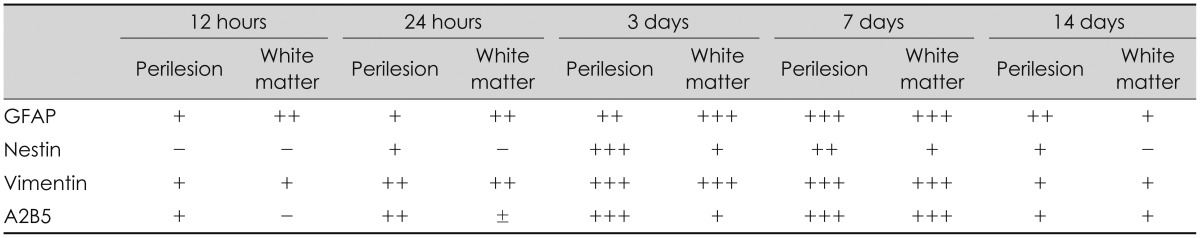
The revelation of the protein over time (Table 1)
GFAP, the marker of a mature astrocyte, is a micro-fiber protein which is the main component of scar tissue in the CNS. An examination was first done 12 hours after the damage, revealing GFAP deep in the damaged white matter, while 24 hours after the damage, there was an increase around the damaged cortex. Seven days after the damage, the revelation of the GFAP reached the highest level. The bilateral edge of the damaged site began to join and continued to show GFAP at seven days after the damage. Finally, at day 14 after the damage, it decreased remarkably (
Figure 1). In the case that the damage could not reach the white matter and became localized in the gray matter, the revelation of the GFAP was increased in the white matter.
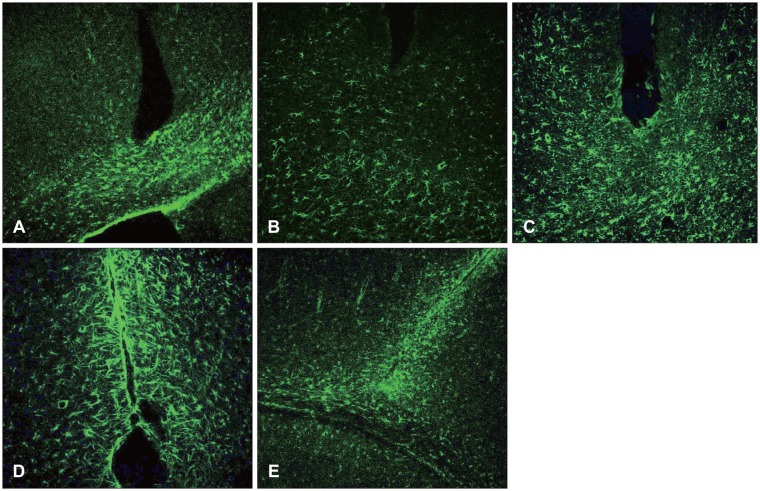 | FIGURE 1Glial fibrillary acidic protein upregulation in white matter; proliferation of astrocytes in white matter at the early stage of scar formation (×100). A: 12 hours. B: 1 day. C: 3 days. D: 7 days. E: 14 days.
|
Nestin was not revealed in the early state (12 hours after damage), and a day later, some was revealed in the vessel walls, but not in the astrocytes. Three days after the surgery, the nestin was increased around the damaged site, and was also revealed in the white matter. Seven days after the surgery, the revelation state was maintained around the nearby cortex and white matter. However, 14 days after surgery, the nestin remained only in the damaged edge, but no longer existed in the nearby white matter and gray matter (
Figure 2).
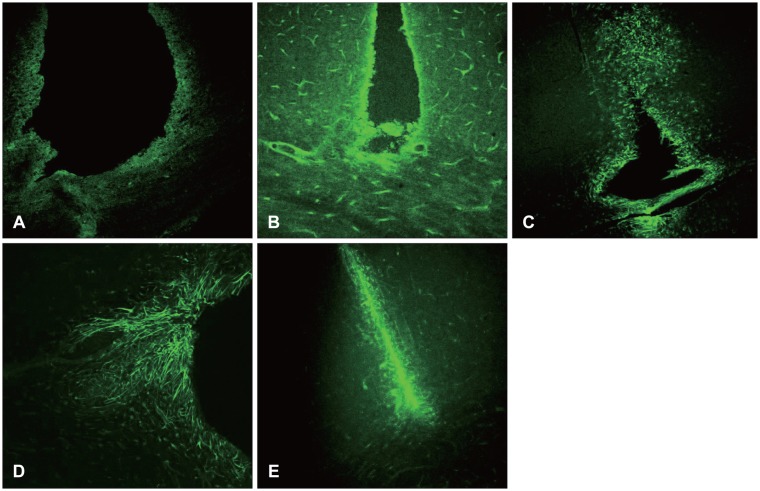 | FIGURE 2Nestin is usually detected in multipotential stem and reactivated astrocytes. This finding suggests that activation and differentiation of astrocytes should be involved in the process of scar formation (×100). A: 12 hours. B: 1 day. C: 3 days. D: 7 days. E: 14 days.
|
Vimentin was revealed 12 hours after the damage and one day after the damage, and was revealed to be increased near the damaged site and white matter. Unlike the nestin, it was also revealed in the early state of damage in the white matter, and was increased until 7 days after the surgery. It was then decreased 14 days after the injury (
Figures 3 and
4).
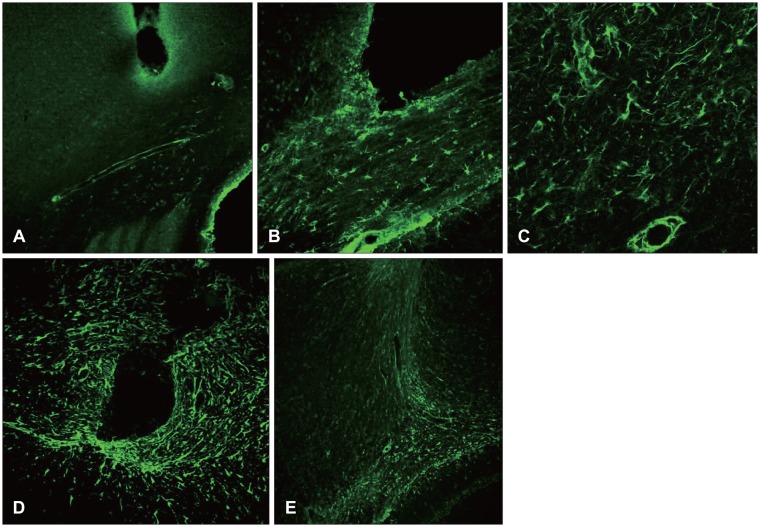 | FIGURE 3Vimentin is an embryonic protein. Its upregulation might be associated with cellular differentiation. It is known that vimentin is involved in cellular motility, so vimentin positive astrocyte might be postulated to migrate from the white matter to the injury site, and to constitute scar later (×100). A: 12 hours. B: 1 day. C: 3 days. D: 7 days. E: 14 days.
|
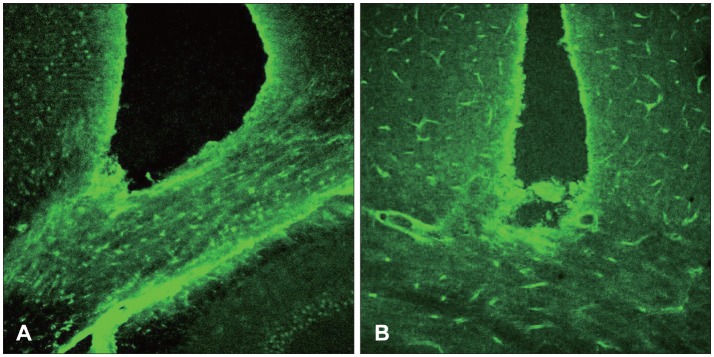 | FIGURE 4A: Vimentin was increased in the white matter. B: Nestin was increased along the wound edge (×100).
|
Some elevation of the A2B5 was seen around the edge of the damaged site after 12 hours, and one day after the surgery it was revealed around the whole area of the damaged fringe. It reached the highest level at 7 days, and decreased remarkably at 14 days after the surgery (
Figure 5).
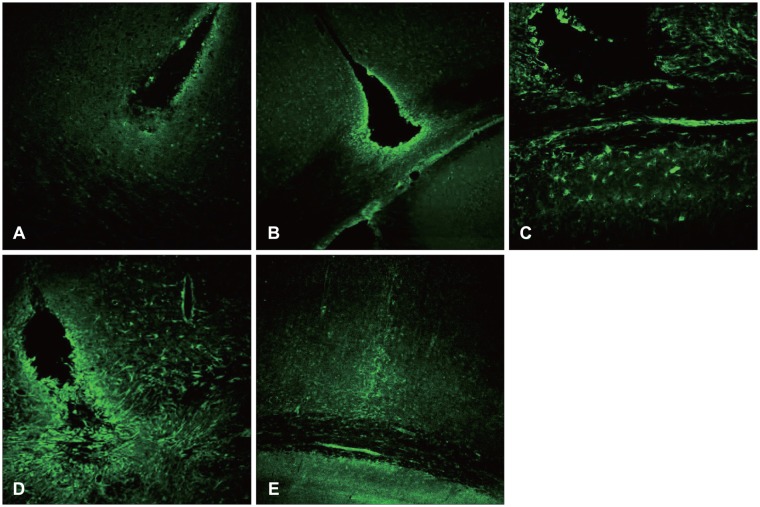 | FIGURE 5A2B5 upregulation surrounding injury site means that the process of activation, differentiation of astrocytes is involved in the glial scar formation (×100). A: 12 hours. B: 1 day. C: 3 days. D: 7 days. E: 14 days.
|
TABLE 1
The expression of the intermediate filament and A2B5

Group revelation (Figure 6)
Seven days after the surgical damage, the nestin, vimentin, and A2B5 were revealed, together with GFAP expression. The group revelation of the nestin, vimentin, and A2B5 was also seen in high resolution (
Figure 6).
In the control rats, the GFAP was expressed in the cells throughout the cerebral cortex. After the injury, more astrocytes were stained for GFAP around the lesion, especially in the white matter. At three days post-injury, the majority of the GFAP positive astrocytes in the white matter underlying the lesion expressed the markers for nestin and vimentin, and the marker for the glial precursor cell A2B5. The number of GFAP positive cells reached a peak on the seventh day post-injury, and then decreased.
1)
Table 1 summarizes the expression of the intermediate filament and A2B5.
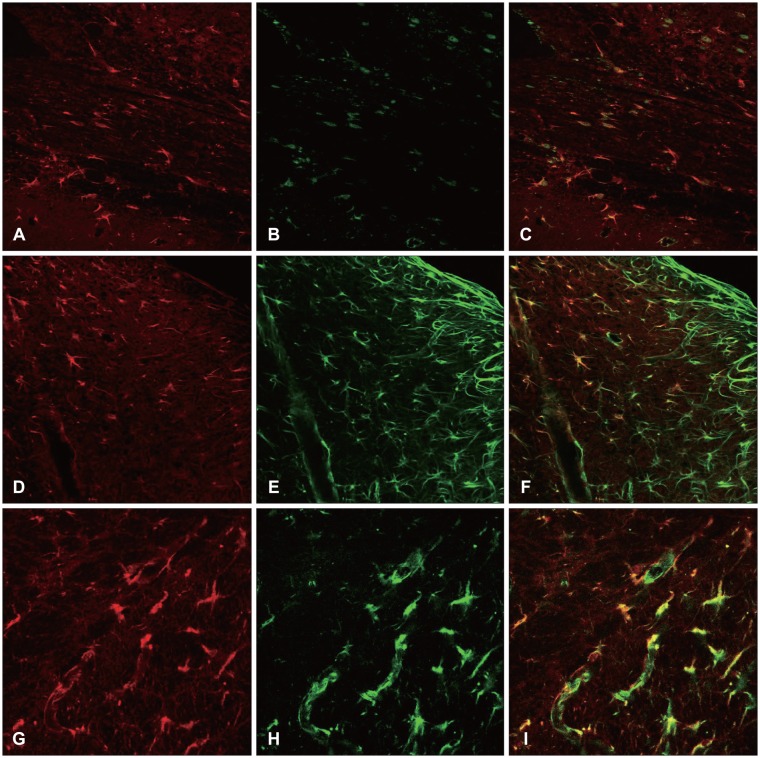 | FIGURE 6Seven days after the surgical damage, the nestin, vimentin, and A2B5 were revealed, together with glial fibrillary acidic protein (GFAP) expression (×100). A-C: Colocalization of GFAP and A2B5. D-F: Colocalization of GFAP and nestin. G-I: Colocalization of GFAP and vimentin.
|
Time based characteristic of protein expression
At 12 hours post-injury, the GFAP began to be expressed in the white matter underlying the lesion, while the vimentin and A2B5 were expressed in both the white and gray matter. The nestin was not expressed.
At 1 day post-injury, the expressions of the GFAP, vimentin, and A2B5 were more increased, and nestin was detected in the area surrounding the wound. While the vimentin was expressed in the underlying white matter, as well as in the perilesional area, the nestin was only expressed in the perilesional area.
At 3 days post-injury, the GFAP expression increased along the edge of the wound, while it decreased in the white matter. It was expressed not only in the area adjacent to the lesion, but also in the area remote from the lesion in the injured hemisphere.
At 7 days post-injury, the separate margins of the lesion were connected, and the scar at the wound edge still had strong staining for nestin, vimentin, and A2B5.
At 14 days post-injury, the expressions of the GFAP, nestin, vimentin, and A2B5 were markedly decreased. The GFAP was co-localized with the nestin, vimentin, and A2B5.
Although the injury was confined to the cortex, the proliferation and activation of the astrocytes occurred in the white matter underlying the lesion,
1) prior to the cortical injury site.
Go to :

Discussion
Glial scar formation is a necessary bio-response of the natural healing process of a wound, but in the CNS, the axon of a damaged neuron creates an unregenerable environment, and it becomes the most important factor in the interruption of the recovery of the nerve function.
141517) Therefore, if one can determine the mechanism of scar formation, and block the process in order to delay the time of scar formation and reduce its range, it may help in the recovery of the damaged nerve functions. Of course, scar formation plays a unique role, which is to recover the original function by isolating it from foreign materials and healing it, and if one blocks this process, additional side effects may occur. However, the CNS is isolated by the skull and vertebrae, and unlike the other organs (like those of the digestive system), it does not have motility. Therefore, if the healing process is somehow delayed, the effects on the original function may not be that great.
The cell which plays the most important role in scar formation after cerebral damage is the astrocyte, and many research studies have been conducted with regard to their increasing processes.
The exact mechanism of glial scar formation is not yet known, but it is assumed that the activation and migration of astrocytes is involved.
891013) However, the origin of the activated astrocytes that contribute to the formation of the glial scar is unclear. One possibility is that the activated astrocytes differentiate from the multipotential progenitor cells and migrate to the injury site.
1220) A source for the progenitor cells that has been identified is the area of the gray matter surrounding the central canal of the spinal cord, but whether other periventricular zones of the brain play similar roles is unknown. Alternatively, the existing astrocytes in the area surrounding the injury site may be activated and proliferate.
711)
One of the most distinct morphological changes in the process of glial scar formation is the increased expression of GFAP in the cells around the injury site. GFAP is a specific cellular marker for differentiated astrocytes, and is the main component of the scar. However, it is present in quiescent, non-reactive astrocytes, so it is not used as a marker for the activation of the astrocytes.
1)
A2B5 is expressed in glial precursor cells and reactive astrocytes.
616) It is a cell surface ganglioside, which was initially detected in the neurons, but later it was also shown to be expressed in non-neural cells, including glial precursor cells and reactive astrocytes.
6) The proliferating oligodendrocyte progenitor cells can differentiate into oligodendrocytes or reactive astrocytes, and both of these cell types express A2B5 during differentiation. The utility of A2B5 as a marker is supported by the fact that it is selective for differentiating glia, and is not expressed in non-reactive astrocytes.
18) A2B5 upregulation surrounding the injury site means that the process of the activation and differentiation of the astrocytes is involved in scar formation.
The embryonic intermediate filaments nestin and vimentin are usually expressed in multipotential stem cells, and they are upregulated in glial cells following injury to the CNS. There is general agreement that nestin and vimentin-upregulating glial cells play a major role in glial scar formation.
2512) Nestin is known as an embryonic protein that is expressed in multipotential stem cells or reactive astrocytes. Vimentin has been reported to serve as a source of cytokines, or as a physical conduit for migrating cells from distant sites.
20) While the nestin was usually expressed in the perilesional area, the vimentin was expressed in the white matter, as well as in the perilesional area. Vimentin-positive astrocytes in the white matter may be associated with the migration of the astrocytes from the white matter into the injury site in the process of scar formation.
1)
In cases where the injury has reached the white matter, the GFAP expression was more increased in the white matter than in the gray matter, and the scar size in the white matter was also larger than that in the gray matter. This may be associated with the fact that GFAP upregulation is more distinct in the white matter than in the cerebral cortex in the early phase of glial scar formation. The GFAP expression was also increased throughout the injured cortex, remote from the lesion.
Douen et al.
5) reported that nestin was detected surrounding the lesion, and throughout the lateral cortex, after cortical ablation. The exact mechanism for this is not yet known, but it might be associated with a global cellular connection.
In this study, the results suggest that the astrocytes are activated and proliferated in the white matter underlying the lesion, and migrate into the wound site, thus constituting a scar. The astrocytes which were in the deep portion were more activated and proliferated than those in the injured cerebral cortex, while the astrocytes in the white matter migrated to the injury site.
In this process, embryonic proteins (nestin and vimentin) are upregulated, and they are involved in the differentiation, proliferation, and migration of astrocytes in the process of scar formation.
Go to :

Conclusion
In the early state after CNS damage, the astrocytes are reactivated in the white matter, which is in the deep part of the damage. Near the site of the damage, the immature cells are differentiated or astrocytes are reactivated to fill in the damaged area, forming scars. In this process, nestin, vimentin, and A2B5 were revealed in the astrocytes, and these factors may be involved in the division, proliferation, and transportation of the astrocytes.
Go to :






 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download