Introduction
The traumatic intracranial pseudoaneurysm (TICPA) is rarely developed following traumatic brain injuries. It happens in about 3% of traumatic brain injuries and consists of less than 1% of the cerebral aneurysms.
15) The symptoms of traumatic intracranial aneurysm are dependent on its location and severity of head trauma. Moreover, it can make usually delayed intracranial hemorrhage (ICH), which may lead to death in more than 50% of the patients.
57) Therefore it is important for the patient's prognosis that managing the traumatic intracranial aneurysm before developing the delayed ICH.
There are many ways to treat the TICPA including aneurysm clipping, trapping of the parent artery, and endovascular coiling. Trapping of the parent artery must be a perfect method not to fail the treatment. But, it may cause the ischemic insult into the offending vascular territory.
2) Furthermore, the trapping of the internal carotid artery (ICA) for the treatment of TICPA in the petrous ICA will induce more serious complications. Balloon test occlusion (BTO) should be preoperatively applied before trapping the ICA. However, unconsciousness can make a difficulty to perform the BTO and the perfusion study. We are to introduce the one-day protocol of single photon emission computed tomography (SPECT) as a perfusion study in an unconscious patient to undergo the ICA trapping for the treatment of traumatic pseudoaneurysm in the petrous ICA.
Go to :

Case Report
A 23-year-old man was admitted with a semicomatose consciousness after getting involved in a traffic accident on driving a car. His Glasgow Coma Scale was 5T (1/T/3) and his right pupil was dilated of 5 mm without light reflex. The right facial palsy was observed. Multiple scalp contusions, the right bloody otorrhea, and nasal bleeding were seen. On the brain computed tomography (CT), there were subarachnoid hemorrhage and airs on the basal cisterns and intraventricular hemorrhage in the 4th ventricle. Sulci in the cerebral hemisphere and cisterns were effaced. And a fracture on the right petrous apex and multiple facial bone injuries were found (
Figure 1). His neurologic status was getting worse and his oculocephalic reflex was absent. An intraparenchymal type of intracranial pressure monitor (Camino™, Integra, Plainsboro, NJ, USA) was indwelled and ventilator with sedation was maintained with massive neurosurgical intensive care. The intracranial pressure was checked as 9 to 10 mm Hg.
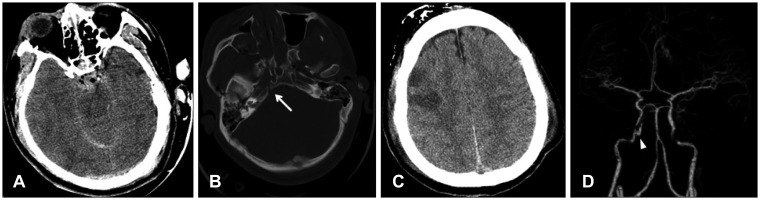 | FIGURE 1Initial brain computed tomography (CT) and CT angiogram. A: Subarachnoid hemorrhage and pneumocephalus on the interpeduncular cistern was seen. B: Multiple facial bone fractures and fracture on the right petrous apex (arrow) nearby the carotid canal were found. C: Multiple hypodense lesions were seen on the right frontal at the 2nd day of trauma. D: Abnormal dye filling sac (arrowhead) at the petrous internal carotid artery was found in the CT angiogram. The pseudoaneurysm was located in the posterior part of cavernous sinus.
|
At the 2nd day of trauma, multiple hypodense lesions on the right frontal lobe were found on the brain CT (
Figure 1). Brain CT angiogram was taken for evaluating the injury of intracranial artery at the 4th day of trauma, which showed an abnormal dye filling sac at the petrous apex (
Figure 1). The pseudoaneurysm was located at the right proximal cavernous ICA around the fracture line. It was irregular ovoid shape with anterior-medial direction in the size of 10×6 mm.
On the next day after the brain CT angiogram, trans-femoral cerebral angiography was done. The pseudoaneurysm grew in size and arterial dissection was also noted at the entrance of the ICA into petrous bone (
Figure 2). We planned to do the trapping of the pathologic ICA and get the perfusion study for evaluate the ischemic tolerance after ICA is sacrificed. We discussed the perfusion study with a specialist of nuclear medicine. She proposed the one-day protocol of SPECT, which is taking twice scans before and after the BTO in the same day. Unfortunately, the patient was sedated and unconscious, so we could not get the neurological changes during the BTO. Twenty mCi of technetium-99m-ethyl cysteinate diethylester (
99mTc-ECD) was injected before the angiogram and then 40 mCi of it was given at 15 minutes in the balloon occlusion of the right ICA. After 40 mCi of it was injected, perfusion SPECT was taken. No change of vital signs was noted during the temporary ICA occlusion. As a result of the SPECT, perfusion asymmetry in the right frontal cortex was aggravated about 21% of perfusion reduction at the post-occlusion scan compared with the baseline scan before the occlusion (
Figure 3). So, trapping of the damaged ICA and the revascularization was mandatory for the definite treatment.
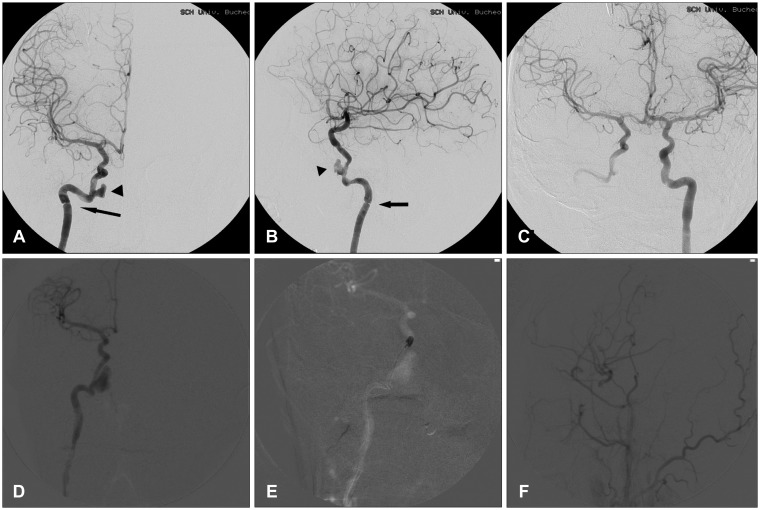 | FIGURE 2Catheter angiogram for the further evaluation of vascular injury. The catheter angiogram was taken the day after the computed tomography angiogram. The pseudoaneurysm (arrowhead) was grown in size. Arterial dissection (arrow) was also found at the entrance of petrous bone and around the anterior part of cavernous sinus (A, B). This vascular injury might be caused by the immobilization of the artery to the petrous bone and the dural ring. The right middle cerebral artery (MCA) was clearly visualized on the right internal carotid artery (ICA) angiogram (C). The pseudoaneurysm size grew markedly in the intraoperative angiogram during the endovascular coiling on the cavernous ICA distal to the pseudoaneurysm (D, E). Intraoperative angiogram after the ICA trapping and the revascularization showed that the distal branches of the right MCA was visualized from the superficial temporal artery (F).
|
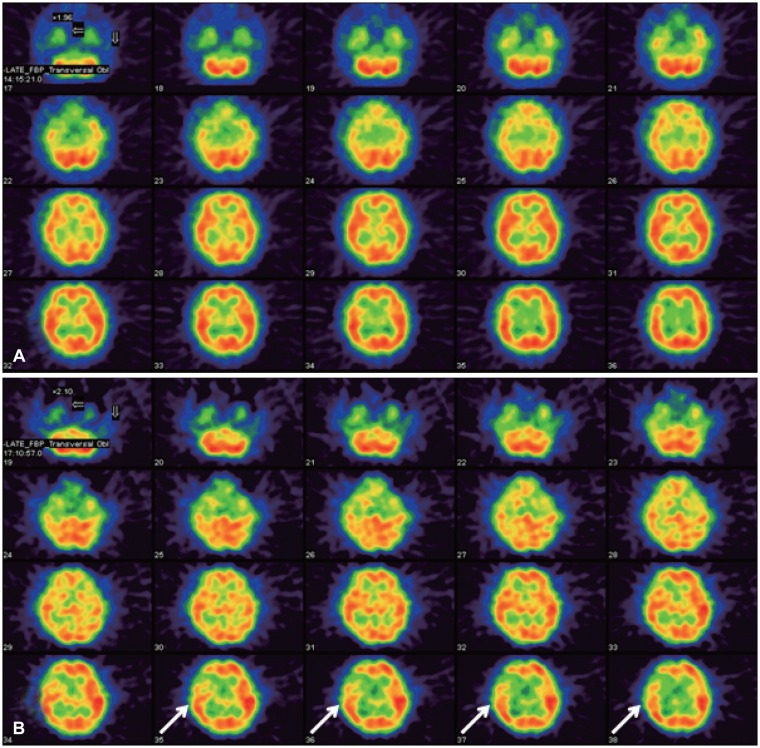 | FIGURE 3Perfusion study of single photon emission computed tomography (CT) before and after balloon occlusion test for the right internal carotid artery (ICA) in the same day. The baseline scan (A) was taken by injecting 20 mCi of technetium-99m-ethyl cysteinate diethylester (99mTc-ECD) before the angiogram and 40 mCi of 99mTc-ECD was given at 15 minutes in the balloon occlusion of the right ICA (B). At the post-occlusion scan, aggravation of perfusion asymmetry on the right frontal cortex was seen, especially in the middle and posterior frontal cortex (arrows) and about 21% of perfusion reduction was measured. The hypoperfusion area in the right frontal was matched with the infarct zone in the brain CT.
|
He got a direct anastomosis of the right superficial temporal artery-middle cerebral artery and ICA trapping between the cervical ICA and the cavernous ICA. Endovascular coiling on the cavernous ICA distal to the pseudoaneurysm was performed in the operating room (
Figure 2). Bypass blood flow from the extracranial to intracranial artery and from the left ICA to the right cerebral arteries was good. After the surgery, he recovered his consciousness and suffered from deafness on the left ear. After six weeks later from operation, he had ventriculo-peritoneal shunt operation for the post-traumatic hydrocephalus. And then he went back his normal life and has not developed any new neurologic sings for 8 years.
Go to :

Discussion
TICPA could be developed from many types of head trauma, especially blunt and penetrating injury.
5) These aneurysms happened rarely as a pseudoaneurysm, which consist of less than 1% of cerebral aneurysms. The mortality was relatively very high in 32% to 54% after the delayed disastrous hemorrhage.
1510) The peak incidence of TICPA has been known at 2 to 3 weeks after the trauma. Therefore, the early diagnosis and management to TICPA is crucial to rescue the patient's life.
As the treatment of TICPAs, surgical and endovascular methods have been proposed.
15) Direct clipping, wrapping or trapping was tried in the surgical methods. And as endovascular method, embolization with detachable coils with/without stent has also been performed. The aneurysm clipping has many advantages that it provides definitive isolation of the aneurysm, allows for the reconstruction of the parent artery if needed, and facilitates removal of mass effect via evacuation of intracranial hematoma and deflation of the aneurysm itself.
5) On the other hand, the use of endovascular therapy avoids prolonged anesthesia, minimizes manipulation of adjacent vessels and structures, and allows diagnostic angiography to be performed throughout the case.
5) But, surgically inaccessible lesion, long-injured segment, and larger proximal aneurysm with high flow like our case should be definitely treated with the trapping all the injured artery.
Before deciding the ICA sacrifice, we should know the ischemic tolerance after the ICA occlusion. ICA trapping makes ischemic stroke in 30% to 40%.
13) Therefore, evaluating cerebral ischemic tolerance before sacrificing ICA is very important to planning treatment strategy. BTO of the ICA can be performed with an acceptably low complication rate with 0.4% of permanent neurologic changes.
9) But, neurologic assessments alone for evaluating ischemic tolerance during BTO have a high false negative rate. ICA sacrifice made cerebral infarction in 5% to 20% even though no neurologic changes happen during BTO.
811) SPECT study has been considered to be the most useful method for evaluating the regional cerebral blood flow (rCBF) during BTO. Twice scans are always needed as the baseline and the blood flow reserve. As the residual activity of radiopharmaceuticals should clear out, the scan takes minimum 2 days.
4) However, these 2 days would be unacceptable for the unconscious patient and emergent situations.
The shorter study time was mandatory for our case with unconsciousness, ventilator applied and unstable condition.
The distribution of
99mTc-ECD did not change in a pattern consistent with rCBF during 16-hour period after injection in animals.
12) It is also report to be stable in human brain for the variety of rCBF.
6) Based on this minimal washout of ethyl cysteinate diethylester (ECD), one-day protocol for cerebral perfusion reserve with acetazolamide was proposed.
3) This protocol is as the below; SPECT scans are obtained after 1st injection of approximately 370 MBq of
99mTc-ECD, 1,000 mg of acetazolamide is given intravenously at 14 minutes after 1st dose injection, and then another SPECT scans are taken after 2nd injection of double dose, approximately 740 MBq of
99mTc-ECD. They concluded that ECD washout was minimal during the first 50 minutes after injection and was not affected by acetazolamide.
3) The distribution of
99mTc-ECD is stable over time, and its washout is independent from rCBF. This is the important characteristics for assessing rCBF changes before and after acetazolamide administration.
3) The 2nd scan images were subtracted with the 1st scan images. In this way, quantitative assessment was possible. In our study, we think that ischemic challenge by performing the temporarily BTO is a substitute for acetazolamide injection. So, double dose injection of
99mTc-ECD and the subtraction method could be a good diagnostic way to evaluate the ischemic tolerance in a short time in less than 1 hour without waiting the complete washout of radiotracer. In our case, we had to decide the treatment method before rupture of the pseudoaneurysm. As it was growing its size and the injured segment of ICA was long, the trapping of ICA would be a definite treatment. So, the ischemic tolerance should be evaluated with BTO and perfusion study with SPECT with urgent. Double-dose injection of
99mTc-ECD would be a good way to know the cerebral perfusion with shorter study time.
Go to :








 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download