This article has been
cited by other articles in ScienceCentral.
Abstract
Few studies have examined the clinical features and long-term outcomes of isolated pituitary hormone deficiencies after traumatic brain injury (TBI). Such deficiencies typically present at time intervals after TBI, especially after mild injuries such as concussions, which makes their diagnosis difficult without careful history taking. It is necessary to improve diagnosis and prevent life threatening or morbid conditions such as those that may occur in deficiencies of adrenocorticotropic hormone (ACTH) or thyroid-stimulating hormone (as known as thyrotropin, TSH), the two most important pituitary hormones in hypopituitarism treatment. Here, we report two cases of isolated ACTH deficiency and one case of isolated TSH deficiency. These patients presented at different time points after concussion and underwent long-term follow-ups.
Go to :

Keywords: Brain injuries, Adrenocorticotropic hormone, Thyrotropin, Hypopituitarism
Introduction
Traumatic brain injury (TBI), a common cause of death and disability among adults, is considered a significant public health problem worldwide. Although pituitary dysfunction caused by TBI was previously considered as rare, accounting for less than 1% of all new cases of hypopituitarism, an increased prevalence has been found in recent years.
5) The pooled prevalence of hypopituitarism between 3 and 12 months after TBI is 27.5%.
9) However, few studies have investigated the prevalence of deficiencies of other pituitary hormones after TBI such as persistent isolated adrenocorticotropic hormone (ACTH) or thyroid-stimulating hormone (as known as thyrotropin, TSH) deficiencies.
The majority of patients develop symptoms of hypopituitarism within 1 year after trauma, most commonly within 6 months.
1) However, 15% of patients are diagnosed 5 or more years after trauma;
3) the maximum reported lag time between trauma and diagnosis of hypopituitarism is 46 years.
3)
Here we report two cases of isolated ACTH deficiency (IAD) and one case of isolated TSH deficiency (ITD), which presented at 10 days, 4 years, and 20 years after head concussion, respectively and were followed-up over a long-term period.
Go to :

Case Report
Cases 1 and 2 are patients with IAD who presented with critically low sodium levels, and case 3 is a patient with ITD who presented with abnormal thyroid function.
Case 1
A 51-year-old man visited our emergency room because of loss of consciousness combined with nausea and vomiting. Ten days prior to this incident, he had fallen 4 meters and sustained a left rib fracture. A brain computed tomography scan had not been performed at that time because he had shown no neurologic signs or symptoms. He did not have any medical history of diabetes, hypertension, or cardiovascular disease, and had never been treated with glucocorticoids. His serum sodium concentration was 105 mmol/L (range, 136 to 146 mmol/L) with normal potassium (range, 3.5 to 5.0 mmol/L), glucose of 50 mg/dL, and morning cortisol of 1.48 µg/dL (range, 5-25 µg/dL) (
Table 1). Brain magnetic resonance imaging (MRI) findings were normal (
Figure 1A). We performed a standard dose ACTH simulation test. In this test, plasma cortisol is measured immediately before, and 30 and 60 minutes after an intravenous injection of 250 µg Synacthen (synthetic ACTH
1-24; Dalim BioTech, Hwaseong, Korea). The patient's cortisol concentration was 1.65 µg/dL at baseline, 3.11 µg/dL at 30 minutes, and 2.91 µg/dL at 60 minutes, with a basal ACTH of 10.02 pg/mL (range, 6 to 76 pg/mL). The patient's other laboratory findings, including basal pituitary hormone concentrations, were unremarkable (
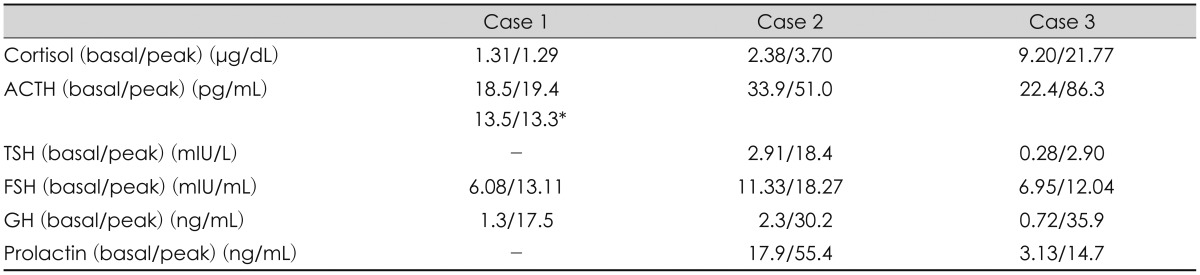
Table 2). His symptoms and mental status dramatically improved with hypertonic saline and glucocorticoid supplementation. We hypothesized that his adrenal insufficiency was related to his fall, and performed a dynamic pituitary stimulation test with corticotropin-releasing hormone (CRH), growth hormone-releasing hormone (GHRH), and luteinizing hormone-releasing hormone (LHRH). Decreased ACTH response to CRH was compatible with isolated ACTH deficiency (
Table 2). For the past 7 years, physiologic doses of glucocorticoid have been necessary to maintain normal biochemical and clinical status.
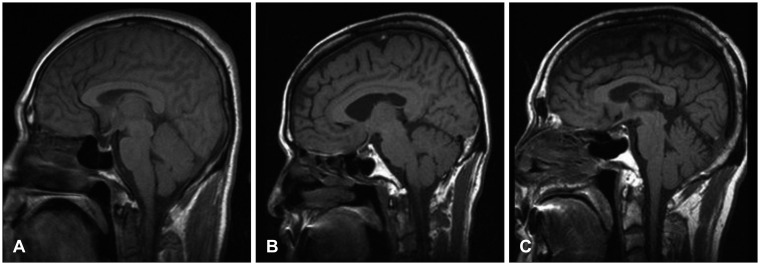 | FIGURE 1T1-weighted sagittal magnetic resonance imaging of case 1 (A), case 2 (B), and case 3 (C) showing a normal pituitary gland.
|
TABLE 1
Clinical characteristics and laboratory findings at presentation
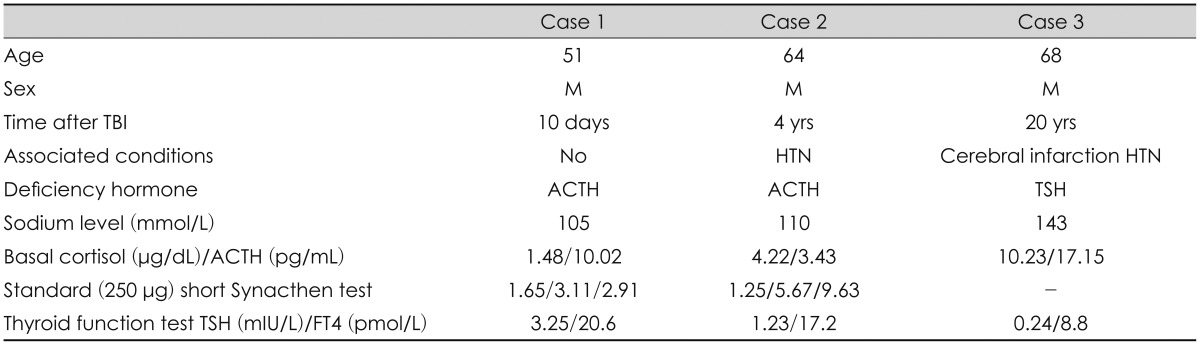

TABLE 2
The results of dynamic tests for pituitary function

Case 2
A 64-year-old-man visited our emergency room with nausea, vomiting, and dizziness after a recent history of upper respiratory infection. The patient had experienced a fall from a height of 3 meters from a stepladder about 4 years prior to presentation. At that time, he suffered from a bilateral visual defect but was never evaluated since the defect was transient and improved without treatment. He also had a history of intramuscular injections at a primary clinic for back pain related to a herniated lumbar disc for 2 years prior to presentation. Serum sodium and potassium concentrations were low (110 and 3.2 mmol/L, respectively), and the serum cortisol level was 4.22 µg/dL. A standard dose ACTH stimulation test was performed. His cortisol concentration was 1.25 µg/dL at baseline, 5.67 µg/dL at 30 minutes, and 9.63 µg/dL at 60 minutes, with a basal ACTH of 3.43 pg/mL (
Table 1). He had been taking a medication for hypertension. Considering his medical history of intramuscular injections, we assumed at the initial examination that his clinical presentation and laboratory abnormalities were related to the more common iatrogenic adrenal insufficiency, since glucocorticoid is often used for these injections. However, the patient did not show clinical features of any glucocorticoid excesses, such as easy bruising or cushingoid appearance, and his degree of hyponatremia was relatively severe. We performed brain MRI to rule out causes of central hypopituitarism, such as tumors. No abnormalities were detected on the MRI scan (
Figure 1B). Prompt glucocorticoid replacement improved the patient's clinical symptoms and hyponatremia. He was maintained on a physiologic dose of glucocorticoid (prednisolone 2.5 to 5 mg). His general condition and electrolyte levels were well maintained during the replacement of physiologic doses of prednisolone. However, he exhibited no recovery regarding basal cortisol and ACTH levels during the long-term follow up period (>1 year), during which he received no glucocorticoid administration except for physiologic doses of prednisolone in the mornings. Considering the patient's history, we suspected TBI-induced IAD and performed a dynamic pituitary stimulation test with CRH, TSH-releasing hormone (TRH), GHRH, and LHRH. The decreased ACTH response to CRH was a finding compatible with IAD (
Table 2). In the 7 years since, the patient has maintained physiologic doses of glucocorticoid and has shown no signs of recovery.
Case 3
A 68-year-old man visited our department because of abnormal thyroid function with tiredness. The patient had been treated for hypertension with amlodipine for 20 years. For around 2 years prior to his visit to our hospital, he had also taken 50 µg/day of levothyroxine at a primary clinic under the suspicion of mild hypothyroidism. Despite this medication, he still experienced tiredness and general weakness. Laboratory findings revealed a TSH concentration of 0.24 mIU/L (range, 0.17 to 4.05 mIU/L) and free T4 of 8.8 pmol/L (range, 11 to 24 pmol/L); anti-microsome Ab was negative at 0.06 U/mL (range, 0 to 0.3 U/mL) (
Table 1). Despite the decrease in free T4 level below the lower normal range (in the absence of non-thyroidal illness), his TSH level did not show an appropriate increase. Central hypothyroidism was thus suspected, and we therefore checked other basal pituitary hormone levels and performed a pituitary MRI scan. However, these did not reveal any abnormalities (
Figure 1C). In combined pituitary stimulation tests with CRH, TRH, GHRH, and LHRH, only TSH response to synthetic TRH was abnormally reduced (
Table 2). ACTH, growth hormone (GH), and gonadotropin responses to their synthetic peptide hormones were normal. These signs were compatible with ITD. ITD remains a very rare disease and is mainly caused by acquired lesions.
6) As this patient had an average adult height and normal mental development, we did not consider it necessary to perform a genetic screening test to rule out congenital central hypothyroidism. Idiopathic ITD was carefully diagnosed after excluding patients with factors affecting the hypothalamo-pituitary-thyroid axis in the absence of structural abnormality in the pituitary MRI image, such as the intake of thyroid function-related medications or the presence of concomitant systemic illness. However, after checking the patient's history in detail, we found that the patient had experienced head trauma. Twenty years prior, a thick board had fallen on his head from 5 meters height at a construction works. He recalled that he felt strong impact to the head and got a painful swelling in the left parietal area for several days, but he did not visited a hospital or received any medical treatment at all. He described that his head trauma was not associated with loss of consciousness or any neurological signs at that time. TBI has been recognized as one of causes for ITD.
6) The levothyroxine dosage was gradually increased. Over a follow up of 3 years and on a maintenance dosage of 100 µg/day of levothyroxine, the patient felt well and maintained a euthyroid state clinically and biochemically.
Go to :

Discussion
An increased prevalence of pituitary dysfunction caused by TBI has been reported in recent years,
5) and so it is crucial that we understand it more fully. To achieve this, we need to examine its relation to TBI, its time course, and patients who may be at higher risk.
While most patients develop hypopituitarism symptoms relatively early, within 1 year after trauma, most commonly within 6 months,
1) there can be a substantial lag time between trauma and diagnosis.
3) The exact mechanism for the delayed time course of the development of post-traumatic isolated pituitary hormone deficiency is thus poorly understood. Indeed, the cases we have described also showed variable lag times; IAD presented immediately or several years after head trauma, and ITD presented decades after head trauma. The relatively longer lag time of our case 3 (20 years) is consistent with other reported cases, with lag times of 36 and 46 years after head trauma in central hypothyroidism.
3)
A trend toward improvement in pituitary function over time has been found, with some of the early abnormalities proving to be transient with complete recovery.
8) However, all three cases described experienced prolonged isolated pituitary hormone deficiency for several years without any signs of recovery. Autoimmunity has been put forward as one mechanism that could promote the occurrence of hypopituitarism following head trauma. For example, one study reported that antipituitary antibodies were found in 46% of subjects who sustained head trauma three years prior to observation, and that this was a predictor for the development of hypopituitarism.
10) The authors assumed that antigens sequestered in the hypothalamic-pituitary tissue pass into the bloodstream and trigger humoral responses that are able to perpetuate neuroendocrine dysfunction.
10) As this is a delayed response, this mechanism would also explain the time course of pituitary insufficiency, which appears months after TBI. One of the disadvantages of the present report is its inability to identify a direct causal relationship between head injury and pituitary hormone deficiency, particularly in cases 2 and 3. Even in the literature, the association between mild brain injury and pituitary hormone deficiency mostly relies on careful history-taking to recover recollections of head trauma, which are often subjective.
2) Accordingly, it will be necessary to identify adequate brain trauma-specific biochemical surrogate markers of pituitary hormone deficiency, such as autoantibodies.
All of our cases were males over 50 years old at the time of presentation. The gender-based difference in incidences of IAD or ITD after TBI have rarely been reported. Nonetheless, males are at higher risk of post-traumatic hypopituitarism at a ratio of 5:1.
2) Common causes of TBI in patients who develop hypopituitarism are motor vehicle accidents, falls, and violence.
7) Recently, combative sports such as boxing, football, and ice hockey have also been thought to be a potential cause of hypopituitarism.
11) The patients with IAD in our cases had experienced falls, and the patient with ITD had experienced head trauma caused by falling objects at work. Environmental factors such as occupation, exposure rate to trauma, and sports may be responsible for the male predominance of incidence reported in the literature. Biological or genetic factors may also play roles in the differential incidence.
The IAD cases presented at the emergency room with severe hyponatremia (serum sodium <115 mEg/mL) due to adrenal insufficiency. In particular, case 1 showed a markedly decreased cortisol response to the standard dose ACTH simulation test performed at the time of presentation, 10 days after TBI. A standard dose ACTH simulation test is extremely useful to examine adrenal reserve in a simple, safe way, even in an emergency care setting, but it cannot rule out adrenal insufficiency in early phases of central hypopituitarism. In cases of hypopituitarism after transsphenoidal surgery, the adrenal response remains intact for weeks to months before deterioration, since adrenal atrophy due to ACTH deficiency is gradual.
4) Therefore, we hypothesize that the onset of deteriorating adrenal response to ACTH in IAD after TBI may be observed earlier than previously believed after pituitary surgery. Exogenous glucocorticoid-induced adrenal insufficiency and IAD have similar lab findings in basal cortisol and ACTH levels, as observed in case 2. Exact history taking, physical examination, and close monitoring of basal hormones are useful for their differential diagnosis.
Go to :

Conclusion
Pituitary hormone deficiencies are not infrequent among TBI survivors. With increasing participation in outdoor activity and sports, such deficiencies may become an important social issue that requires consensus about follow-up guidelines after TBI, even if they are mild concussions. Our three cases with long-term follow-up suggest the need for a better understanding of clinical characteristics and an elucidation of the pathophysiologic mechanism underlying isolated pituitary hormone deficiency after TBI. More active communication between doctors who care for victims of brain trauma should be encouraged to consider endocrine dysfunction as a potential post-traumatic complication.
Go to :


