This article has been
cited by other articles in ScienceCentral.
Abstract
Multiple methods and materials are available for bone defect reconstruction. Bone graft substitute is one of the materials used for reconstruction of bone defect and have been widely used recently. This report describes some cases about complications related to GeneX® which is introduced as mixture of calcium sulfate and β-tricalcium phosphate at manufacturer's official web site. It informed of 3 patients who suffered wound inflammation, serous cyst after using GeneX® for reconstructing skull defect.
Go to :

Keywords: Skull defect, Bone substitutes, Postoperative complications
Introduction
Skull defect is caused by trauma, tumor, congenital deformity and post operation defect in neurosurgery field. Reconstruction of skull defect is need for cerebral protection, biomechanical stability and improvement of cosmetic deformity.
8) Multiple materials are used for skull reconstruction. Nowadays, bone graft substitutes are used increasingly as alternative to autograft and allograft because of their shortcomings.
211)
GeneX® (Biocomposites Ltd., Staffordshire, UK) is introduced as one of bone graft substitutes. Some studies reported about complications related to GeneX® using for bony reconstruction on various parts.
17) However, complications of GeneX® using for skull reconstruction is not reported yet. GeneX® was used recently to patients who had post-operative skull defect for reconstruction in our hospital. We observed some complications related to GeneX®.
Go to :

Case Report
Case 1
A 62-year-old female had skull mass on right parietal bone. She was underwent operation for removal of skull mass and reconstruction of defect on skull with 10 cc of GeneX®. Nine days after the operation, she complained of neglecting on left side of the body and dizziness. Brain magnetic resonance image (MRI) and brain computed topography (CT) scan were checked for evaluation of the neurologic deficit. There was round cystic lesion in right parietal parenchyme under the operation site in brain MRI (
Figure 1A), and brain CT scan shows loss of the reconstructed material along the inner side (
Figure 1B). Infectious signs such as fever and myalgia were not appeared, and blood levels of white blood cell, erythrocyte sedimentation rate, and C-reactive peptide belonged normal limits. She was underwent operation for removal of the reconstructed material and the cyst with irrigation. In operative field, turbid fluid collection was observed around reconstructive material, and the cyst was seen under the defect site, and it was enclosed in a whitish membrane and collected turbid fluid with many tiny whitish particles inside (
Figure 1C). Reconstruction of the defect was done by using mini-plate and contourable mesh plate. Removed materials from the lesion were referred for histologic examination, and it was identified to acute inflammation (
Figure 1D).
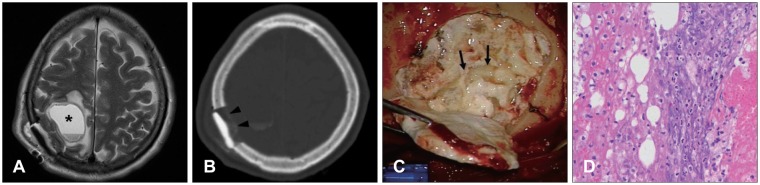 | FIGURE 1A: Brain MRI shows round cyst on right parietal lobe (asterisk). B: Brain computed tomography shows loss of the reconstructed material along the inner side (black arrowheads). C: Photograph shows turbid fluid with multiple tiny particles (black arrows). D: Inflammatory cells are mainly composed of neutrophil (hematoxylin-eosin, original magnification, ×400).
|
Case 2
A 70-year-old woman with a history of breast ductal cell carcinoma and lung metastasis visited due to scalp mass. In physical examination, somewhat hard mass was checked under subcutaneous tissue. Heterogeneous enhanced mass with osteolysis around was found on left frontal bone in enhanced brain CT scan. She was underwent operation for removal of the mass, and 10 cc of GeneX® was used for skull reconstruction. The mass was identified to metastasis on left frontal bone. One month later, she complained about swelling and local pain on previously operative site (
Figure 2A). There was no definite systemic or local infectious sign in laboratory test. There was cystic lesion instead of reconstructed material on follow up brain CT scan (
Figure 2B). She was underwent operation of wound revision and irrigation. In operative field, there was cyst which contained many whitish particles assumed to GeneX® on skull reconstruction site (
Figure 2C). Newly reconstruction was done with using contourable mesh plate. After operation, her discomfort was resolved. In histologic examination, it was identified to chronic inflammation (
Figure 2D).
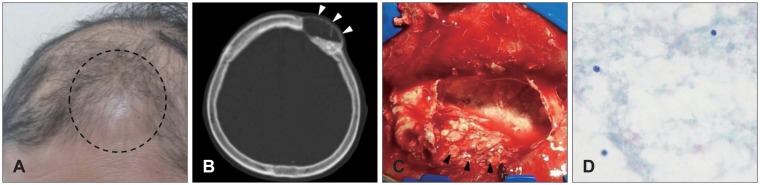 | FIGURE 2A: Swelling on previous operation site is seen (dotted line). B: Follow up brain computed tomography shows cystic lesion instead of reconstructed material (white arrowheads). C: Photograph shows cyst which is enclosed in a whitish membrane and collected turbid fluid with multiple tiny particles inside (black arrowheads). D: Lymphocytes are scattered (hematoxylin-eosin, original magnification, ×400).
|
Case 3
A 49-year-old woman had skull defect on left frontal bone after undergoing surgery of craniotomy and aneurysmal clipping for spontaneous subarachnoid hemorrhage due to ruptured anterior communicating artery aneurysm. She felt cosmetic complex about depression along the inferior margin of temporal line of frontal bone and protrusion at the inferior portion of temporal fossa. Skull reconstruction was done in such a way that 5 cc of GeneX® filled along the previously craniotomy gap and fat graft was done around the depression site. Two months later, there was developed swelling, fluid collection, and local pain on operative site. Fluid aspiration was performed on swelling site. The aspirated fluid looked turbid brown, and whitish particles generated from GeneX® were observed in the fluid. There was no definite systemic or focal infectious sign. She suffered from delayed wound healing. After daily wound dressing was done for a month, her local inflammation was healed. Depression of the inferior margin of temporal line of frontal bone and protrusion at the inferior portion of temporal fossa were remained.
Go to :

Discussion
Many materials have been developed for reconstruction of bony defect. It is divided largely into autologous bone, allologous bone and bone graft substitutes. Autograft is recognized to be gold standard, because it provides osteoconductive, osteoinductive, osteogenic properties.
2) But, autologous bone has complications with donor site morbidity, such as donor site pain, infection.
11) Allologous bone is another considerable choice, but allograft has risk for transferring viral disease, nonunion and immunologic response.
2) Because of those shortcomings, bone graft substitutes are used as alternative, and new products are developing even now.
Bone graft substitutes have variety in composition, mechanism of action. GeneX® is introduced as "absorbable, osteoconductive, synthetic bone graft material with a negatively charged surface to accelerate bone growth in trauma, spine and non-unions" at manufacturer's official web site (
www.biocomposites.com). It composed of calcium sulfate and β-tricalcium phosphate (βTCP) in a same ratio.
10) βTCP have porous with vary in size, and it is similar with trabecular. When bone tissue attached to βTCP surface, osteoid grows and is mineralized, and it turns to bone tissue, and it resorbs at finally.
2) Precise mechanism that calcium sulfate acts as osteoconductive has not been discovered.
9) Some articles reported that calcium sulfate stimulate new bone formation and dissolve rapidly. According to the official web site, GeneX® has advantage that is negatively charged surface to enhance cell attachment and proliferation compared with untreated calcium phosphate. It is injected to fill the bone defect site, and sets within 15 minutes at normal body temperature. Yang et al.
10) presented their animal research which filled with GeneX® to vertebral body defect site of sheep, and they represented their experimental results that GeneX® facilitate osteogenesis more rapidly compared with not using GeneX® group.
Although GeneX® has effective to bone reconstruction, complications relate to GeneX® also reported. Two papers described complications which occurred after the use of GeneX®. Saadoun et al.
7) reported 3 patients who suffered complications related of GeneX® after using for spinal fusion. They also supported their experience by representing mouse experimental results that GeneX® caused significant muscle and adjacent tissue necrosis at injected site. Friesenbichler et al.
1) reviewed 31 patients retrospectively who underwent bone graft with GeneX® after bone tumor removal in orthopedic department, 5 of the 31 patients had developed complications related to GeneX®. Their patients suffered from the complications which commonly involved sterile inflammation, delayed wound healing, local pain and inflammatory cystic formation. They commonly reported marked local aseptic inflammation and inflammatory cystic formation after using GeneX®.
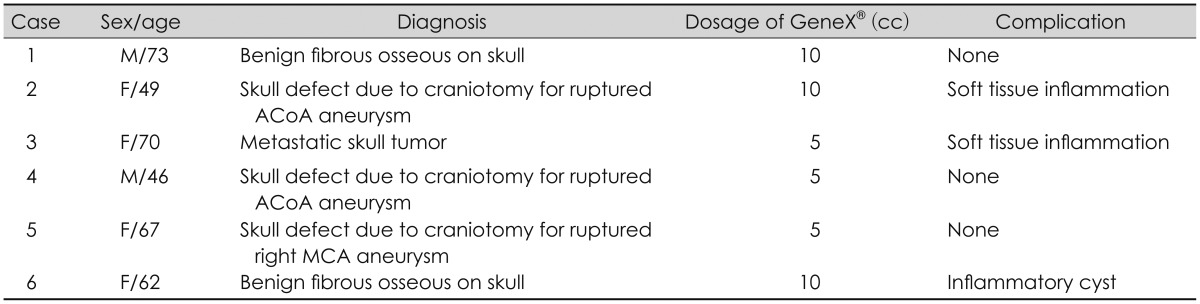
17) In our institutes, GeneX® was used for skull reconstruction to 6 patients in the last 2 years (
Table 1). Half of them exhibited complications related to GeneX® such as wound swelling or inflammatory cyst containing the fluid and whitish particles assumed GeneX®. All of complicated patients suffered from self-limited local inflammation, not systemic infection. It was considered foreign-body reaction, because the lesions were localized to GeneX® graft sites and all of the patients were healed with removing of the material without using antibiotics.
TABLE 1
Information of patients

Both βTCP and calcium sulfate are believed to safe to man. But, some articles reported complications of using calcium sulfate. Most frequent complication is persistent serous drainage from wounds.
3) But, there was no definite pathophysiologic explanation about mechanisms which led to inflammatory reaction. Some hypotheses about mechanism of the inflammation are suggested. One of them is that accumulation of calcium-rich fluid which is derived from too early graft resorption induced inflammation.
56) Another one is that response to osmotic effect caused by not dissolved calcium phosphate.
45) Based on our experiences, it is postulated that fluid collection on dead space by early resorption of GeneX® particle before bone regeneration may cause inflammation to adjacent tissue. Also, other materials such as blood or cerebrospinal fluid can pool into the dead space, and it may inhibit bone regeneration and cause the local inflammation.
Go to :

Conclusion
Bone graft substitute is one of the options for skull reconstruction. It is developing now and new products are introduced. GeneX® is launched recently, so data about efficacy and problem of the products are short. It is easy to use for bone void filler, but early resorption of GeneX® before bone regeneration can induce serous cyst, local inflammation. So, attention is required in case of using GeneX® to large bone defect which needs long time to bone formation. Especially, more attention is need for using GeneX® adjacent to nervous system such as skull can cause neurologic deficit by the local inflammation and the cyst. In addition, broad study is required about safety and complication of GeneX®.
Go to :







 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download