Abstract
Objective
In most cases, the postoperative course of a chronic subdural hematoma (CSDH) is good, but CSDHs sometimes remain after the inserted catheter is removed, and the outcomes of such remnant hematomas are unclear. As oral streptokinase-streptodornase (OSS) has anti-inflammatory and hematologic effects, we assessed the effects of OSS on remnant CSDHs through a retrospective analysis of clinical data.
Methods
This study included 101 patients with traumatic CSDH who had remnant CSDH after burr-hole trephination with the closed drainage system between October 2009 and December 2012. We assessed the clinical outcomes, remnant CSDH volume, and recurrence rate from computed tomography scans in order to evaluate the effects of OSS.
Results
No significant differences were found in the changes in remnant hematoma volume between the OSS-treated and non-treated groups (p=0.531). The reoperation rate because of was 5.4% (2 patients) in the OSS-treated group and 6.3% (4 patients) in the non-treated group. The number of patients requiring reoperation did not differ between the groups (p=0.658).
Go to : 
A chronic subdural hematoma (CSDH) is a common intracranial mass that can be treated using a burr-hole surgery with closed system drainage. In most cases, the postoperative course is good, but the hematoma occasionally remains after the inserted catheter is removed, and the outcomes of such remnant hematomas are unclear. Rohde et al.14) reported that the prognosis of remnant CSDH after surgical treatment was unclear, and frequent recurrence has been reported, in 5% to 33% of patients.24)
Oral streptokinase-streptodornase (OSS) has both anti-inflammatory and useful hematologic effects. It removes clotted blood and fibrinous accumulations of exudates41015) and has been proven to cure hemothorax and intramuscular hematoma.71519) Therefore, we considered examining the effects of OSS on remnant CSDH after surgical treatment. This formed the basis of the present study, in which we retrospectively analyzed clinical data regarding the effects of OSS on remnant CSDH.
Go to : 
This retrospective study included 101 patients with traumatic CSDH who had remnant hematomas after burr-hole trephination with the closed drainage system between October 2009 and December 2012. Remnant hematoma was defined as a CSDH of thickness exceeding 2 mm after the inserted catheter was removed.
We have used OSS for some time, and studied the effect of OSS on remnant CSDH, retrospectively. All cases were divided into the OSS-treated and non-treated groups. The OSS prescription was based on each neurosurgeon's discretion. No etiologic or clinical differences were noted between the groups. OSS was used until completely absorbed time of remnant CSDH in the OSS-treated group.
Indications for surgery included 1) CSDHs with neurological deficits, 2) those with a midline shift of ≥5 mm, and 3) those whose thickness exceeded that of the skull bone.
With the patient under local anesthesia with lidocaine, a scalp incision was made on the parietal eminence. A small burr-hole was made with an electric craniotome on the parietal bone. After dural tack-up sutures were placed, the dura was incised in a cruciate shape. A drainage catheter was quickly inserted into the subdural hematoma space for delayed drainage. The CSDH showed good flow through the drainage catheter. The drainage catheter was tunneled subcutaneously and then fixed to the scalp and connected to the blood bag.
Brain computed tomography (CT) was performed on postoperative days 3, 7, 14, and 21. The catheter was removed when the CSDH was found to be completely cleared on brain CT. We ensured that the period of catheter dwelling did not exceed 3 weeks, in order to avoid infection. After the catheter was removed, all patients underwent follow-up brain CT every month for at least 3 months after surgery.
We compared factors age, sex, and hospitalization day between the groups. The volume of the remnant CSDH was ascertained by integrating the areas measured on the brain CT scans using ImageJ 1.49 (National Institute of Health, Bethesda, MD, USA). Recurrence rate was evaluated from the number of reoperation cases. The effects of OSS were determined on the basis of decreasing remnant volume and CSDH recurrence rate.
All data were analyzed using PASW statistics 18 release 18.0.0 (SPSS Inc., Chicago, IL, USA). The Student t-test and Fisher's exact test were used to compare between-group differences. Repeated-measures analysis of variance was performed to analyze changes in the volume of remnant CSDH. The statistical significance level was set at p<0.05.
Go to : 
This study included 68 men (67.3%) and 33 women (32.7%) whose mean age was 66.3±11.8 years. The mean catheterization time was 9.1±4.3 days. All patients showed neurological improvement after operation.
The number of patients in the OSS-treated group was 37, while that in the non-treated group was 64. These groups showed no significant differences in age (69.8±12.3 years vs. 64.5±10.3 years), hospitalization day (14.7±6.4 days vs. 16.5±5.8 days), or catheterization time (9.87 days vs. 9.03 days).
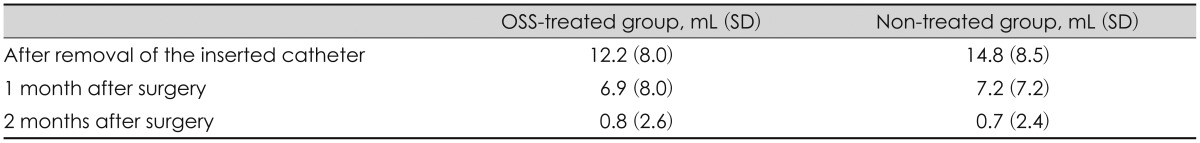
Additionally, the groups showed no significant differences in the changes in the volume of the remnant hematoma (p=0.531). After the inserted catheter was removed, the hematoma volumes in the OSS-treated and non-treated groups were 12.2±8.0 mL and 14.8±8.5 mL, respectively, while those of the remnant CSDH 1 month later were 6.9±8.0 mL and 7.2±7.2 mL, respectively, and 2 months later were 0.8±2.6 mL and 0.7±2.4 mL, respectively (Table 1).
Six patients (5.9%) required reoperation for recurrent CSDH. The reoperation rate because of recurrent CSDH was 5.4% (2 patients) in the OSS-treated group and 6.3% (4 patients) in the non-treated group. The number of patients requiring reoperation did not differ between the groups (p=0.658).
Go to : 
Although CSDH may resolve spontaneously, non-treatment can worsen neurological conditions.11) Among patients with a large CSDH, neurological recovery is better for those who undergo drainage than for those who do not.18) Therefore, CSDH causing neurological deficits should be treated surgically. Current surgical treatments include burr-hole surgery with closed system drainage, burr-hole irrigation without drainage, and craniotomy with inner membrane excision. Ducruet et al.6) reported that the reoperation rate for burr-hole craniotomy with or without closed drainage was 8% to 33% and that for craniotomy with inner membranectomy was 12% to 33%. Furthermore, other studies found that the recurrence rate for burr-hole trephination with closed drainage was lower than that for burr-hole irrigation.21721)
Postoperative outcomes are generally complete resolution or recurrence. Oh et al.13) reported that CSDH completely cleared within 1 month in 38% patients, within 3 months in 42% patients, and after 3 months in 7% patients. Persistent hematomas after surgery are liquefied and encapsulated by membranes. It is difficult to predict whether incompletely removed CSDHs are absorbed or progress to recurrence.21)
Insufficient re-expansion of the brain is considered the key reason for postoperative CSDH recurrence.912) Other reasons are old age, a separated type of CSDH, intracranial hypotension, anticoagulant therapy, and inappropriate timing of operation.158162023) Several measures have been assessed to prevent recurrence, including corticosteroids, angiotensin receptor antagonists, upright head positioning, and continuous postoperative subdural irrigation,1322) but evidence on the efficacy of these measures is limited.
Streptokinase-streptodornase is an enzyme secreted by several species of streptococci that can bind and activate human plasminogen. OSS can dissolve blood clots and purulent necrosis and has been used to treat hemothorax, hematomas, and various chronic suppurations.47101519)
Although we hypothesized that OSS could eliminate remnant CSDHs after surgery and reduce recurrence rates, our findings indicated that OSS had no effect on the prognosis of remnant CSDHs after surgical removal. There was no reasonable basis about the effectiveness of OSS treatment.
The use of OSS may occasionally result in allergic reactions like urticaria, rash, fever and anaphylaxis. But these complication were rare, and were not seen in our case.
A limitation of this study was its small sample size. Further studies with a larger sample size are needed to confirm our results.
Go to : 
References
1. Abouzari M, Rashidi A, Rezaii J, Esfandiari K, Asadollahi M, Aleali H, et al. The role of postoperative patient posture in the recurrence of traumatic chronic subdural hematoma after burr-hole surgery. Neurosurgery. 2007; 61:794–797. discussion 797PMID: 17986941.

2. Alcalá-Cerra G, Young AM, Moscote-Salazar LR, Paternina-Caicedo A. Efficacy and safety of subdural drains after burr-hole evacuation of chronic subdural hematomas: systematic review and meta-analysis of randomized controlled trials. World Neurosurg. 2014; 82:1148–1157. PMID: 25118059.

3. Berghauser Pont LM, Dammers R, Schouten JW, Lingsma HF, Dirven CM. Clinical factors associated with outcome in chronic subdural hematoma: a retrospective cohort study of patients on preoperative corticosteroid therapy. Neurosurgery. 2012; 70:873–880. discussion 880PMID: 21937933.
4. Calandre EP, Ruiz-Morales M, Lopez-Gollonet JM, Hernandez MA, Guerrero JA, Ródenas E, et al. Efficacy of oral streptokinase-streptodornase in the treatment of ankle sprains. Clin Orthop Relat Res. 1991; 263:210–214. PMID: 1899636.
5. Chon KH, Lee JM, Koh EJ, Choi HY. Independent predictors for recurrence of chronic subdural hematoma. Acta Neurochir (Wien). 2012; 154:1541–1548. PMID: 22653496.

6. Ducruet AF, Grobelny BT, Zacharia BE, Hickman ZL, DeRosa PL, Anderson K, et al. The surgical management of chronic subdural hematoma. Neurosurg Rev. 2012; 35:155–169. discussion 169PMID: 21909694.

7. Hettich R, Schunack G. [Reabsorption of standardised intramuscular haematomas in rabbits treated with streptokinase per os (author's transl)]. Arzneimittelforschung. 1981; 31:306–308. PMID: 7194647.
8. Jeong SI, Kim SO, Won YS, Kwon YJ, Choi CS. Clinical analysis of risk factors for recurrence in patients with chronic subdural hematoma undergoing burr hole trephination. Korean J Neurotrauma. 2014; 10:15–21.

9. Jung YG, Jung NY, Kim E. Independent predictors for recurrence of chronic subdural hematoma. J Korean Neurosurg Soc. 2015; 57:266–270. PMID: 25932293.

10. Katavisto M. Streptokinase-streptodornase in the treatment of ulcus serpens corneae. Acta Ophthalmol (Copenh). 1956; 34:214–217. PMID: 13354339.

11. Markwalder TM. Chronic subdural hematomas: a review. J Neurosurg. 1981; 54:637–645. PMID: 7014792.

12. Mori K, Maeda M. Surgical treatment of chronic subdural hematoma in 500 consecutive cases: clinical characteristics, surgical outcome, complications, and recurrence rate. Neurol Med Chir (Tokyo). 2001; 41:371–381. PMID: 11561347.

13. Oh HJ, Lee KS, Shim JJ, Yoon SM, Yun IG, Bae HG. Postoperative course and recurrence of chronic subdural hematoma. J Korean Neurosurg Soc. 2010; 48:518–523. PMID: 21430978.

14. Rohde V, Graf G, Hassler W. Complications of burr-hole craniostomy and closed-system drainage for chronic subdural hematomas: a retrospective analysis of 376 patients. Neurosurg Rev. 2002; 25:89–94. PMID: 11954771.

15. Rutter PM, Carpenter B, Hill SS, Locke IC. Varidase: the science behind the medicament. J Wound Care. 2000; 9:223–226. PMID: 11933332.

16. Sakakibara F, Tsuzuki N, Uozumi Y, Nawashiro H, Shima K. [Chronic subdural hematoma--recurrence and prevention]. Brain Nerve. 2011; 63:69–74. PMID: 21228450.
17. Santarius T, Kirkpatrick PJ, Ganesan D, Chia HL, Jalloh I, Smielewski P, et al. Use of drains versus no drains after burr-hole evacuation of chronic subdural haematoma: a randomised controlled trial. Lancet. 2009; 374:1067–1073. PMID: 19782872.

18. Sarnvivad P, Chiewchanvechakul W, Chumnanvej S. Chronic subdural hematoma: drainage vs. no drainage. J Med Assoc Thai. 2011; 94:1352–1356. PMID: 22256475.
19. Sherry S, Tillett WS, Read CT. The use of streptokinase-streptodornase in the treatment of hemothorax. J Thorac Surg. 1950; 20:393–417. PMID: 14774891.

20. Song DH, Kim YS, Chun HJ, Yi HJ, Bak KH, Ko Y, et al. The predicting factors for recurrence of chronic subdural hematoma treated with burr hole and drainage. Korean J Neurotrauma. 2014; 10:41–48.

21. Wakai S, Hashimoto K, Watanabe N, Inoh S, Ochiai C, Nagai M. Efficacy of closed-system drainage in treating chronic subdural hematoma: a prospective comparative study. Neurosurgery. 1990; 26:771–773. PMID: 2352594.

22. Weigel R, Hohenstein A, Schlickum L, Weiss C, Schilling L. Angiotensin converting enzyme inhibition for arterial hypertension reduces the risk of recurrence in patients with chronic subdural hematoma possibly by an antiangiogenic mechanism. Neurosurgery. 2007; 61:788–792. discussion 792-793PMID: 17986940.

23. Yoshii K, Seki Y, Aiba T. [Causative factors of recurrence of chronic subdural hematoma]. No Shinkei Geka. 1987; 15:1065–1071. PMID: 3323930.
24. Yu GJ, Han CZ, Zhang M, Zhuang HT, Jiang YG. Prolonged drainage reduces the recurrence of chronic subdural hematoma. Br J Neurosurg. 2009; 23:606–611. PMID: 19922274.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download