Abstract
Objective
Traumatic pseudoaneurysms are rare but life-threatening lesions. We investigated the patients with these lesions to clarify their clinical characteristics and therapeutic strategies and we also reviewed the literatures on the treatment principles, possible options, and outcomes.
Methods
There were a total of 8 patients who were treated with traumatic intracranial pseudoaneurysms between April 1980 and January 2009. Medical charts and the imaging studies were reviewed for analysis. The outcome was measured with modified Rankin Scale (mRS) score at 6 months after treatment.
Results
All 8 patients were male and the mean age was 25 years old. Six of those were located at the cavernous segment of the internal carotid artery (ICA) and the other 2 was located at the M2 segment of middle cerebral artery. The causes of trauma were car accidents in 6, penetrating injury through the orbit in 1, and slip down injury in 1 patient. Massive epistaxis or hematemesis occurred in all patients with a pseudoaneurysm at the cavernous and ophthalmic segment of the ICA. All 6 patients of the cavernous and ophthalmic ICA group showed favorable outcome of mRS 0 to 1. The outcome of patients with middle cerebral artery pseudoaneurysm was mRS 2 to 3.
Go to : 
Traumatic intracranial pseudoaneurysms are rare, occurring in less than 1% of patients with cerebral aneurysms.8) Traumatic pseudoaneurysms are often called a pulsating hematoma, which forms when the arterial wall is ruptured by trauma and bleeding is confined only by the adventitia or surrounding tissues.17) After a few days, the clot undergoes fibrous organization and is excavated by the arterial flow, then progressively becomes the sac of a pseudoaneurysm.17) In contrast to the physical structure of a true aneurysm, which has all anatomical layers (intima, media and adventitia), the wall of a traumatic intracranial pseudoaneurysm is composed mainly of blood clot and a little fibrous tissue.17) We investigated the patients with these lesions to clarify their clinical characteristics and therapeutic strategies and we also reviewed the literatures on the treatment principles, possible options, and outcomes.
Go to : 
We retrospectively reviewed the clinical medical records and imaging studies of the 8 patients who were treated with traumatic pseudoaneurysms between 1980 and 2009 at our institute. Management outcome was measured with modified Rankin Scale (mRS) score at 6 months after the endovascular or surgical treatment.
Go to : 
There were 8 patients, all of which were male and mean age was 25 years. 6 of those were located at the cavernous segment of internal carotid artery (ICA) and the other 2 was located at the M2 segment of middle cerebral artery. Table 1 summarizes the demographics and clinical features of all 8 cases.
Massive epistaxis or hematemesis occurred in all patients with a pseudoaneurysm in the cavernous or ophthalmic segment. All 6 patients showed skull base fracture and pneumocephalus on initial brain computed tomography (CT).
All cases occurred after trauma, mostly car accidents (six cases). Of the other 2 cases, 1 was after a penetrating injury through the orbit, the other was after slip down injury. Five of our 8 cases presented with massive epistaxis (4) or hematemesis (1). Change of mentality was the main symptom in the other 3 patients, although epistaxis was accompanied in 1 of the 3 patients. All patients except the one caused by a penetrating wound had basal skull fracture and traumatic subarachnoid hemorrhage. In 6 of 8 patients, the pseudoaneurysm was located in the proximal intracranial ICA, from the cavernous to ophthalmic patient. The other 2 was located at the proximal middle cerebral artery. Five of 6 patients with ICA pseudoaneurysm went through occlusion of the ICA with detachable balloons. The remaining 1 patient received open ligation of the ICA through pterional approach. Collateral circulation through the anterior and posterior communicating artery was evaluated prior to occlusion in 5 patients. In 1 patient, preoperative collateral circulation evaluation was not possible due to irritability of the patient. In this case, we confirmed the collateral circulation after balloon occlusion of the pseudoaneurysm and ICA was done. Of the 2 patients with pseudoaneurysm located in the middle cerebral artery, one underwent craniotomy and clipping of the aneurysm and the other received aneurysmectomy. In total 8 cases, 6 patients had a pseudoaneurysm at the cavernous ICA and the other 2 at the middle cerebral artery. All 6 patients of the cavernous and ophthalmic ICA group showed excellent outcome of mRS 0 to 1. The patients with middle cerebral artery pseudoaneurysm showed a fair outcome of mRS 2 to 3.
The characteristic findings of the cases in our series are described as follows.
In our first case, we performed surgical ligation of the ICA proximal to the sac. A test occlusion procedure was done prior to ligation, and the patient was tolerable and suffered only blurry vision of the affected eye which was present from admission.
In cases 2 to 6, we performed balloon occlusion of the ICA. In 4 of the 5 cases, prior to balloon occlusion, temporary occlusion of the affected ICA was done under electroencephalogram (EEG) monitoring to ensure collateral circulation and also compression tests were done to check whether sufficient flow was seen through the anterior and posterior communicating arteries. We used a total of 3 detachable balloons. The first balloon was detached proximal to the pseudosac and a second balloon was detached at the petrous ICA. The last balloon was detached right above the carotid bifurcation. There was no epistaxis after embolization. Cervical spine and skull X-rays were taken after the patient was stabilized and before discharge to ensure the detached balloons has not migrated and was stable.
In case 2, there was a 2 month interval between trauma and massive epistaxis. Upon initial admission, the patient shown skull base fracture, pneumocephalus and epidural hematoma at the left temporal pole with subarachnoid hemorrhage, but no intracranial vessel study was done. The patient received open reduction and internal fixation due to a fibular head fracture and was discharged. While being admitted at another hospital for conservative care, the patient experienced minimal epistaxis intermittently, but no further evaluation was done. Massive epistaxis occurred suddenly during gentle ambulation and was transferred to our hospital. Angiography revealed a pseudoaneurysm proximal to the ophthalmic branch. Balloon occlusion was performed and the patient was discharged with no serious deficits.
In case 3, the patient showed epistaxis 15 days after trauma, while attempting gradual sitting-up. Angiography revealed a pseudoaneurysm at left cavernous ICA and balloon occlusion was performed as above. Upon occlusion, the patient developed mild sensory change around the face at V1 area, but soon disappeared and no other neurologic deficits have developed.
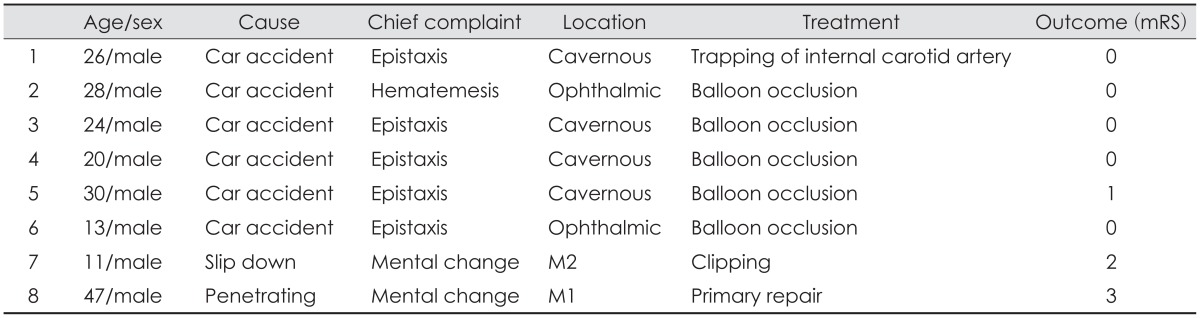
In case 4, epistaxis occurred 7 days after trauma. Upon angiography, pseudoaneurysm at the C3 cavernous portion (Figure 1), a traumatic carotid cavernous fistula (CCF) and dissecting aneurysm of the middle meningeal artery was seen. After balloon occlusion was done as above, additional coil embolization of the middle meningeal artery was performed.
In case 5, the patient has admitted via outpatient department presenting intermittent epistaxis after trauma (Figure 2). Upon angiography and balloon occlusion, the patient showed drowsy mentality and severe dysarthria, probably due to an embolism by thrombus in the aneurysmal sac. Motor grade was intact, and we sedated and intubated the patient. We were unable to perform test occlusion and compression tests in this patient prior to occlusion. Upon post-occlusion angiography, sufficient collateral circulation was identified and minimal retrograde filling of the pseudoaneurysm sac was seen. Because the retrograde flow was very slow and minimal, we expected the pseudosac to become completely obstructed due to thrombus formation and did not perform any additional intervention. The patient rapidly recovered from the dysarthria and drowsiness next day. The patient was discharged with minimal palsy of the left 6th nerve.
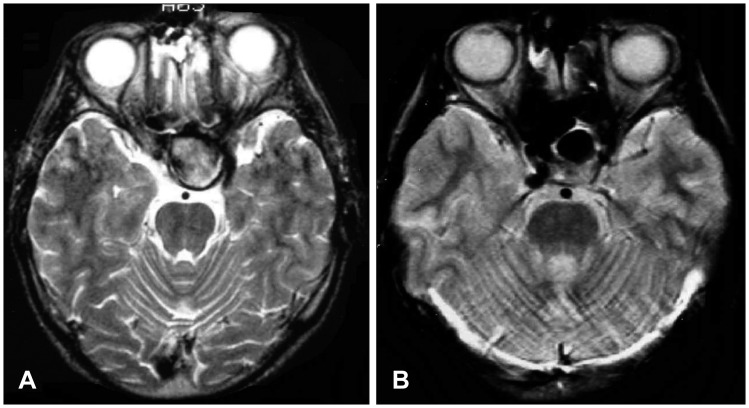
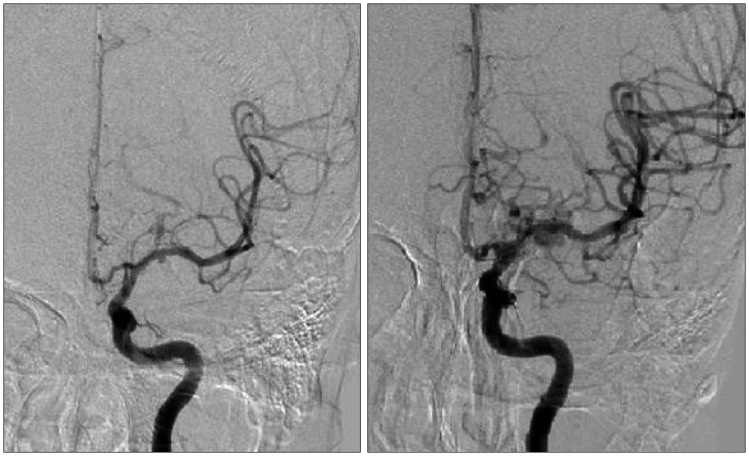
In case 6, epistaxis developed 6 weeks after trauma. Angiography showed a pseudoaneurysm at the right cavernous ICA (Figure 3A). After identification of sufficient collateral flow and retinal blush, balloon and coil occlusion of the parent artery as above was done (Figure 3B and C). The patient developed no new neurologic deficits, and was discharged with right side blindness which was present from initial admission.
In case 7 and 8, the pseudoaneurysm was located at the middle cerebral artery. Unlike those at the cavernous ICA, the patients did not show epistaxis and there was no sign of skull base fracture on CT scan. Both cases showed mental deterioration as the main symptom.
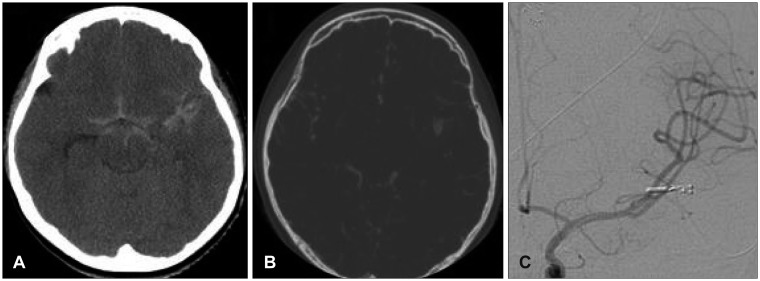
In case 7, the patient developed markedly progressing mental deterioration upon hospital arrival. Initially he was nearly alert, but was stuporous when the CT scan was taken. A pseudoaneurysm was seen at CT angiography (Figure 4A and B) and we performed emergency craniectomy. Upon sylvian dissection after hematoma evacuation, we noticed a saccular formation most likely a pseudoaneurysm and clipping was done. There was no residual sac filling seen on postoperative angiography (Figure 4C). After postoperative medical care, the patient nearly fully recovered mentality and was discharged for further rehabilitation. Upon follow-up, he still suffers from intermittent headaches and grade 4 right hemiparesis.
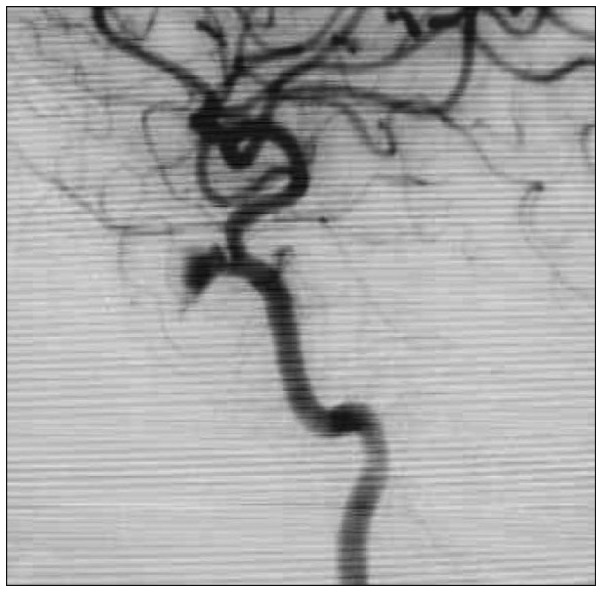
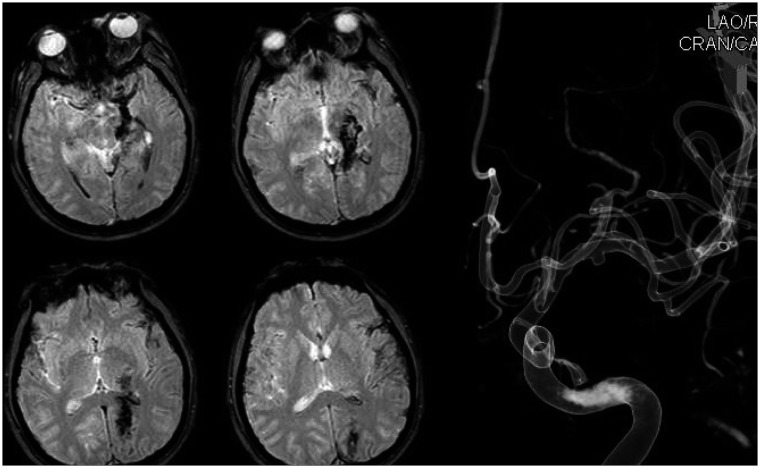
In case 8, the patient had arrived with mental change after a penetrating injury through his left orbit. A rigid wire has penetrated through his left eye and coursed to the occipital lobe, as we could see findings in his CT and magnetic resonance imaging scans which suggest brain contusion along the penetrating pathway (Figure 5). We did not perform immediate surgery but provided medical care with increased intracranial pressure control, because the pseudoaneurysm visible on initial catheter angiography was small, and we concluded the rupture risk was rather low. After 1 month, the patient's neurological status has stabilized and follow-up angiography was done, revealing increased size of the pseudoaneurysm (Figure 6). The patient underwent craniotomy and upon sylvian dissection, we could see a pseudoaneurysm of the M1 segment of middle cerebral artery and after application of a temporary clip, aneurysmectomy with primary repair of the parent vessel was done. The patient's initial motor weakness of grade 1 at right extremities have recovered to grade 3 upon discharge. Also, visual field exams showed homonymous hemianopsia of the right visual field, most likely due to injury of the left occipital lobe.
Go to : 
Traumatic pseudoaneurysms of the cavernous segment of the ICA are related with the classic triad of symptoms: massive epistaxis, unilateral blindness and skull base fracture.3) In these cases, severe and life-threatening epistaxis may occur weeks to months, even years after head trauma. Massive epistaxis following head trauma is a rare presentation.3) Some of the possible underlying cause may be a CCF, a pseudoaneurysm of the cavernous carotid artery (CCA), transsphenoidal surgery or any other injury of the ICA and its branches in approximation to the basal skull and paranasal sinuses and also radiosurgery.315) The epistaxis is massive in these cases, and may be life threatening.1361013) Also, unilateral vision loss is common due to hemorrhage in the cavernous sinus.13) Angiographic study of the carotid artery is indicated in these cases with a presentation of subarachnoid hemorrhage, basal skull fracture and epistaxis.3) But the event of epistaxis may occur several weeks to months later after trauma, and thus diagnosis may be delayed. This delayed diagnosis may contribute to the high morbidity, mortality of patients with these lesions.3)
Aneurysms located at the cavernous portion of the ICA are rare, and rarely need treatment due to lower risk of rupture. Cases treated are mostly large and giant sized aneurysms with symptoms due to compression of the cranial nerves.6) Traumatic cavernous ICA pseudoaneurysms are also rare, but require definite treatment due to a higher rupture and re-bleeding risk. The higher risk is mainly because of two reasons: the wall of a pseudoaneurysm is histologically weaker and thus more susceptible to rupture, and pseudoaneurysms are once ruptured aneurysms, by definition.
Treatment of aneurysms in this location is challenging. Surgical approach to the cavernous sinus carries significant risk of injury and/or dysfunction of the cranial nerves. On the other hand, endovascular approach to this region is relatively easy and is an excellent treatment option for aneurysms of the cavernous portion of the ICA. But unlike an idiopathic aneurysm where a true aneurysmal wall exists, a pseudoaneurysm does not have a true wall and cannot be packed with detachable coils.3) Thus the more applicable treatment option is total occlusion of the effected ICA. Approximately 75% of all patients are tolerable to unilateral ICA occlusion3) and develop no neurologic deficits, thus the collateral flow of all patients that are scheduled to undergo occlusion of the ICA must be evaluated prior to treatment.16) The most widely performed test is the Matas test, in which the ICA is temporarily occluded with an inflatable/deflatable balloon while a neurologist continues to check the neurological status of the patient under EEG monitoring. Patients that fail to pass the test should be considered as a candidate of bypass surgery, such as the superficial temporal artery to middle cerebral artery bypass.3)
In our cases, all 6 patients received therapeutic occlusion of the effected ICA-1 received surgical trapping and the other 5 went through endovascular balloon occlusion. Although occlusion of the parent vessel was a treatment of choice in the past, there has been many reports of successful treatment of these lesions by remodeling the vessel wall using flow diverter stents25) or a combination of stent placement and stent assisted coil embolization.49) Because a flow diverter stent limits blood flow to end-vessel like lesions; thus an aneurysm, true or false, and can be performed in patients that do not have sufficient collateral flow, it may become more effective in treating these lesions in the future. But there are limitations in flow-diverter placement, such as a minimal diameter of the vessel it is placed.
In our case series, all patients except one where balloon test occlusion was not possible due to unstable fluctuating mental status, has passed the test occlusion and no post-procedural ischemic events were seen. The one case in which balloon test occlusion was not possible prior to treatment, collateral flow was adequate on post-procedural angiograms. Temporary balloon test occlusion of the parent artery is a well-accepted method for assessing ability to tolerate permanent occlusion of the vessel.16) All 6 patients showed no newly developed permanent neurological deficits due to ischemia during follow-up.
Traumatic pseudoaneurysms of the middle cerebral artery are also rare, and a few cases have been reported.7111214) It may be caused by any head trauma, blunt or penetrating. These pseudoaneurysms commonly present with intracranial hematoma. Like traumatic pseudoaneurysms at other locations, treatment is required due to high risk of re-bleeding. Although therapeutic occlusion of the parent vessel is a valid treatment option in those with adequate collateral vascular supply, it may be challenging as the diameter of the vessel is smaller and tortuous vascular anatomy may make superselection difficult.7) Although treatment of pseudoaneurysms of the middle cerebral artery is not documented as well as those of the cavernous ICA, it shares the same principles: occlusion of the parent artery is more effective due to the histological nature of these lesions. Selection of whether an endovascular or surgical approach may depend on the location of the aneurysm.
We reviewed 2 cases of traumatic pseudoaneurysms of the middle cerebral artery and both patients underwent surgical treatment. In our case no. 8, we were able to primarily repair the ruptured wall of the parent vessel, but it may be challenging in many cases according to the location of vessel wall tear.3) The aneurysm in our case no. 7 may have been a true aneurysm, as we were able to apply a clip at the aneurysm neck, although we did not harvest any aneurysmal wall tissue for a pathological diagnosis. In both cases, the outcome was fair and no major complications were seen throughout follow-up.
Go to : 
Traumatic intracranial pseudoaneurysms occur likely in young age males, as in our case series. In traumatic pseudoaneurysms located at the cavernous ICA, balloon occlusion of the parent artery is an effective treatment in patients that have sufficient collateral flow. Traumatic pseudoaneurysms at the middle cerebral artery may be treated under the same principles, depending on the location and other vascular anatomy of the patient. The outcome and prognosis is favorable if proper treatment is provided following prompt diagnosis of such lesions. In our opinion, endovascular treatment including balloon occlusion and graft stent or flow diverter placement seems more suitable for pseudoaneurysms of the CCA, and lesions of the middle cerebral artery favors surgical treatment, such as clipping, trapping and primary repair.
Go to : 
References
1. Adeel M, Ikram M. Post-traumatic pseudoaneurysm of internal carotid artery: a cause of intractable epistaxis. BMJ Case Rep. 2012; 5. 23. 2012:[Epub]. DOI: 10.1136/bcr.02.2012.5927.
2. Amenta PS, Starke RM, Jabbour PM, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, et al. Successful treatment of a traumatic carotid pseudoaneurysm with the pipeline stent: case report and review of the literature. Surg Neurol Int. 2012; 3:160. PMID: 23372976.

3. Bavinzski G, Killer M, Knosp E, Ferraz-Leite H, Gruber A, Richling B. False aneurysms of the intracavernous carotid artery--report of 7 cases. Acta Neurochir (Wien). 1997; 139:37–43. PMID: 9059710.
4. Cohen JE, Gomori JM, Segal R, Spivak A, Margolin E, Sviri G, et al. Results of endovascular treatment of traumatic intracranial aneurysms. Neurosurgery. 2008; 63:476–485. discussion 485-486PMID: 18812959.

5. Giorgianni A, Pellegrino C, Minotto R, Mercuri A, Frattini L, Baruzzi F, et al. Flow-diverter stenting of post-traumatic bilateral anterior cerebral artery pseudoaneurysm: a case report. Interv Neuroradiol. 2015; 21:23–28. PMID: 25934771.

6. Honeybul S, Barker S, Poitelea C, Ditchfield A. Multiple intracranial aneurysms presenting with epistaxis. J Clin Neurosci. 2006; 13:394–397. PMID: 16540324.

7. Horiuchi T, Nakagawa F, Miyatake M, Iwashita T, Tanaka Y, Hongo K. Traumatic middle cerebral artery aneurysm: case report and review of the literature. Neurosurg Rev. 2007; 30:263–267. discussion 267PMID: 17440757.

8. Larson PS, Reisner A, Morassutti DJ, Abdulhadi B, Harpring JE. Traumatic intracranial aneurysms. Neurosurg Focus. 2000; 8:e4. PMID: 16906700.

9. Lim YC, Kang JK, Chung J. Reconstructive stent-buttressed coil embolization of a traumatic pseudoaneurysm of the supraclinoid internal carotid artery. Acta Neurochir (Wien). 2012; 154:477–480. PMID: 22187050.

10. Maldonado-Naranjo A, Kshettry VR, Toth G, Bain M. Non-traumatic superior hypophyseal aneurysm with associated pseudoaneurysm presenting with massive epistaxis. Clin Neurol Neurosurg. 2013; 115:2251–2253. PMID: 23932468.

11. Nathoo N, Nadvi SS. Traumatic intracranial aneurysms following penetrating stab wounds to the head: two unusual cases and review of the literature. Cent Afr J Med. 1999; 45:213–217. PMID: 10697918.

12. Ohta M, Matsuno H. Proximal M2 false aneurysm after head trauma--case report. Neurol Med Chir (Tokyo). 2001; 41:131–134. PMID: 11372556.
13. Ruiz-Juretschke F, Castro E, Mateo Sierra O, Iza B, Manuel Garbizu J, Fortea F, et al. Massive epistaxis resulting from an intracavernous internal carotid artery traumatic pseudoaneurysm: complete resolution with overlapping uncovered stents. Acta Neurochir (Wien). 2009; 151:1681–1684. PMID: 19350203.

14. Santos G, Lima T, Pereira S, Machado E. Traumatic middle cerebral artery aneurysm secondary to a gunshot wound. J Neuroimaging. 2013; 23:115–117. PMID: 21223435.

15. Tuchman A, Khalessi AA, Attenello FJ, Amar AP, Zada G. Delayed cavernous carotid artery pseudoaneurysm caused by absorbable plate following transsphenoidal surgery: case report and review of the literature. J Neurol Surg Rep. 2013; 74:10–16. PMID: 23943714.

16. Wang Q, Chen G, Gu Y, Song D. Provocative tests and parent artery occlusion in the endovascular treatment of distal middle cerebral artery pseudoaneurysms. J Clin Neurosci. 2011; 18:1741–1743. PMID: 21992743.

17. Wang X, Chen JX, You C, He M. Surgical management of traumatic intracranial pseudoaneurysms: a report of 12 cases. Neurol India. 2008; 56:47–51. PMID: 18310837.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download