Abstract
Objective
The risk factors for surgical site infections (SSIs) after cranioplasty following decompressive craniectomy remain unclear. The goal of this study was to analyze the risk factors related to developing SSIs after cranioplasty and to suggest valuable predictors.
Methods
A retrospective review was conducted of patients who underwent cranioplasty following decompressive craniectomy at our institution from January 2011 to December 2014, a total of 78 patients who underwent 78 cranioplasties. Univariate and multivariate logistic regression analyses were carried out to determine possible risk factors related to developing SSIs. We analyzed both patient-specific and surgery-specific factors.
Results
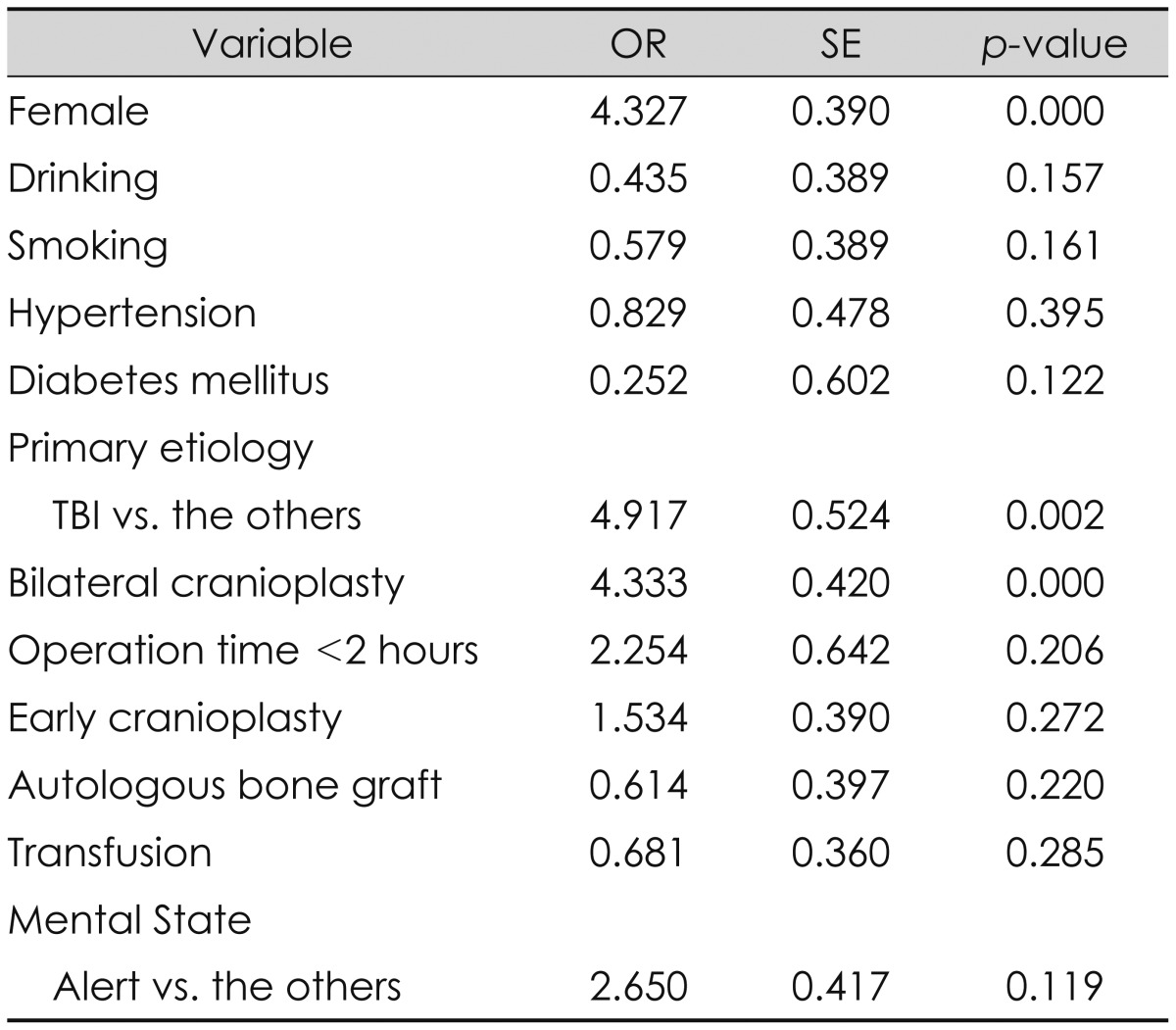
The overall rate of SSIs was 9.0% (7/78). SSIs after cranioplasty were significantly related to being female, having the primary etiology of traumatic brain injury (TBI) and having had a bilateral cranioplasty in the univariate analysis. Multivariate logistic regression analysis showed that being female [odds ratio (OR) 5.98, p=0.000] and having had a bilateral cranioplasty (OR 4.00, p=0.001) significantly increased the risk of SSIs.
Decompressive craniectomy is a neurosurgical life-saving option for treating medically refractory increased intracranial pressure (ICP), such as from severe traumatic brain injury (TBI), poor-grade aneurysmal subarachnoid hemorrhage (aSAH) or malignant cerebral infarction. Its efficacy is currently being investigated with respect to survival and functional outcomes in the various settings related to increased ICP.410222324) For patients who survive after decompressive craniectomy, subsequent cranioplasty is usually performed for cosmetic reconstruction of the skull defect. Additionally, cranioplasty may help to improve neurocognitive functioning by restoring brain hemodynamics.1811) However, surgical site infections (SSIs) after cranioplasty can reduce functioning because of the removal of the bone flap. Many studies emphasize the technical aspects of surgery to reduce SSIs after cranioplasty, such as the interval between decompressive craniectomy and cranioplasty or the type of flap materials.56131518) Recent studies, however, emphasize the patient-specific oversurgery-specific factors as major predictors of SSIs after cranioplasty.2830) There are relatively few clinical studies about this issue, and the risk factors for SSIs remain unclear.
In the current study, we retrospectively analyzed the risk factors related to developing SSIs in patients with refractory increased ICP who underwent cranioplasty after decompressive craniectomy. Further, we suggest valuable predictors by validating the risk factors that were identified in previous studies.
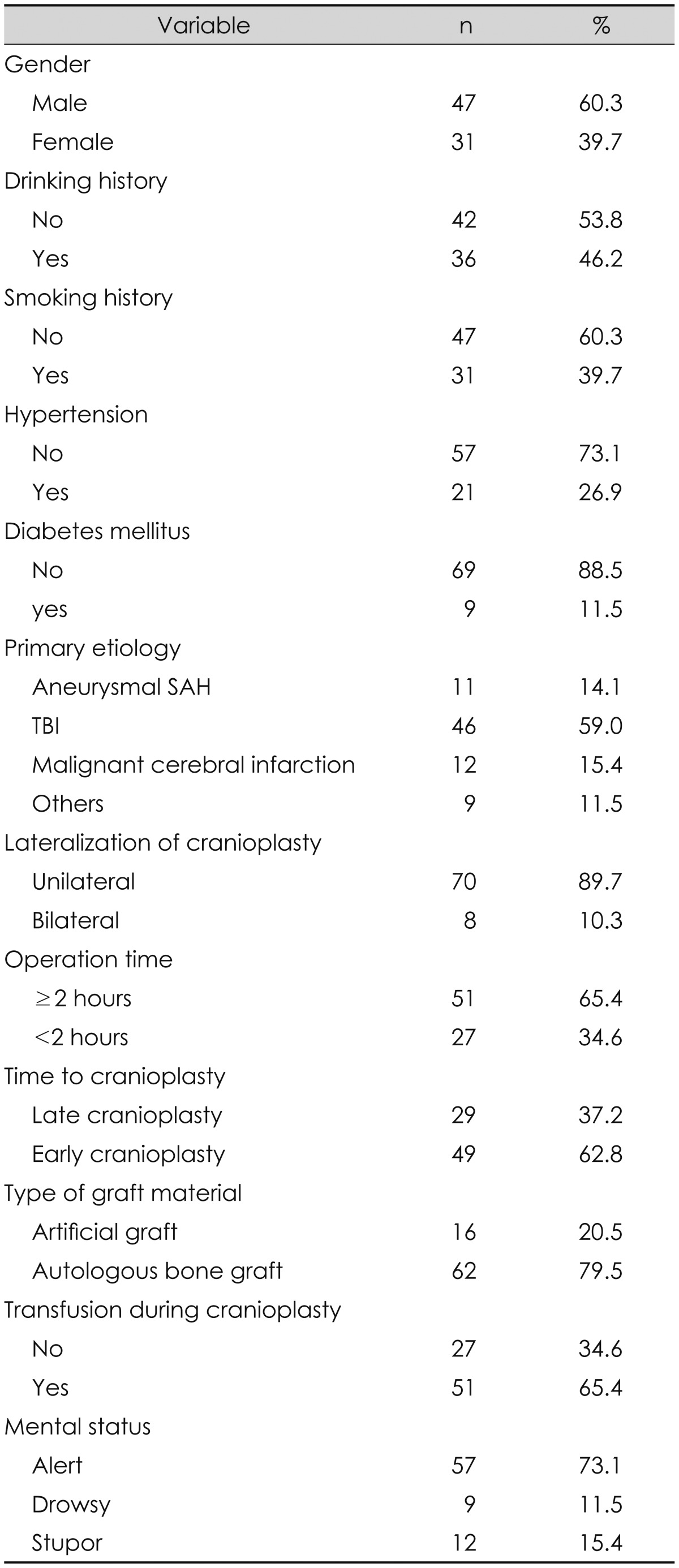
From January 2011 to December 2014, 122 patients underwent cranioplasty at our institution after decompressive craniectomy. Patients were excluded if they 1) had insufficient follow-up data; 2) underwent other operations combined with the cranioplasty; or 3) underwent simple craniectomy without increased ICP, such as for infectious disease or craniofacial anomalies. Ultimately, 78 patients who underwent 78 cranioplasties were selected for analysis with approval from our Institutional Review Board. The mean age was 53.0 years at the time of the cranioplasty (range, 10 to 78). Forty-seven patients (60.3%) were male and 31 (39.7%) were female. The mean interval between the decompressive craniectomy and the cranioplasty was 116 days (range, 14 to 1,347). After the cranioplasty, the mean follow-up period was 16.4 months (range, 0.5 to 51). All patient data were retrospectively collected using hospital chart reviews.
All patients underwent standard cranioplasty. The edges of the skull were trimmed to match the flap material using a drill and a diamond burr. The flap was fixed to the skull using a cranio fix and/or the mini-plating system.
The autologous bone flap was preserved and sterilized using the following regular techniques according to our policy. After the decompressive craniectomy, the bone flap was preserved in a deep freezer at a temperature of -75℃. Connective tissues such as muscles, the fascia and the galea were entirely removed before the freezing. The autologous bone flaps were then packed in a two-layer sterile instrument pouch. Ethylene oxide gas sterilization of the cryopreserved bone material was conducted in the deep freezer, and it was additionally sterilized by high-pressure steam before the graft implantation. The sterile instrument pouch was opened, and the bone flap was placed in a sterile Vancomycin-/Betadine solution for 30 minutes or so before the incision during the cranioplasty. If the autologous bone flap was unavailable because of the etiology of the cranial defect, such as contaminated bone caused by an open head injury or severely comminuted bone, prosthetic material was used. Two grams of prophylactic intravenous cefazolin were given on induction, and 2 g of postoperative intravenous cefazolin were given every 24 hours for 7 days.
Patient demographic data including age and gender were collected, in addition to the following factors: history of smoking and drinking, hypertension (HTN), and diabetes mellitus (DM), primary etiology, type of graft material (autologous bone vs. artificial graft), lateralization of cranioplasty (unilateral vs. bilateral), operating time for the cranioplasty (within 2 hours or not), mental status at cranioplasty, time to cranioplasty after decompressive craniectomy (early or late cranioplasty), and blood transfusion during cranioplasty. The primary etiology was defined as the reason for the decompressive craniectomy and categorized as aSAH, TBI, malignant cerebral infarction or other. Early cranioplasty was defined as surgery performed within 3 months. The patients' variables are summarized in Table 1. SSI after cranioplasty was defined as a graft infection that required removing the graft material.
Analysis was performed using the chi-square independence test or independent t-test as appropriate. Continuous variables are expressed as mean±standard deviation. Univariate and multivariate logistic regression analyses were performed to determine the variables associated with SSIs following cranioplasty. Statistical significance was accepted at probability values of less than 0.05. These analyses were performed using IBM SPSS statistics software version 20.0 (SPSS Inc., Chicago, IL, USA).
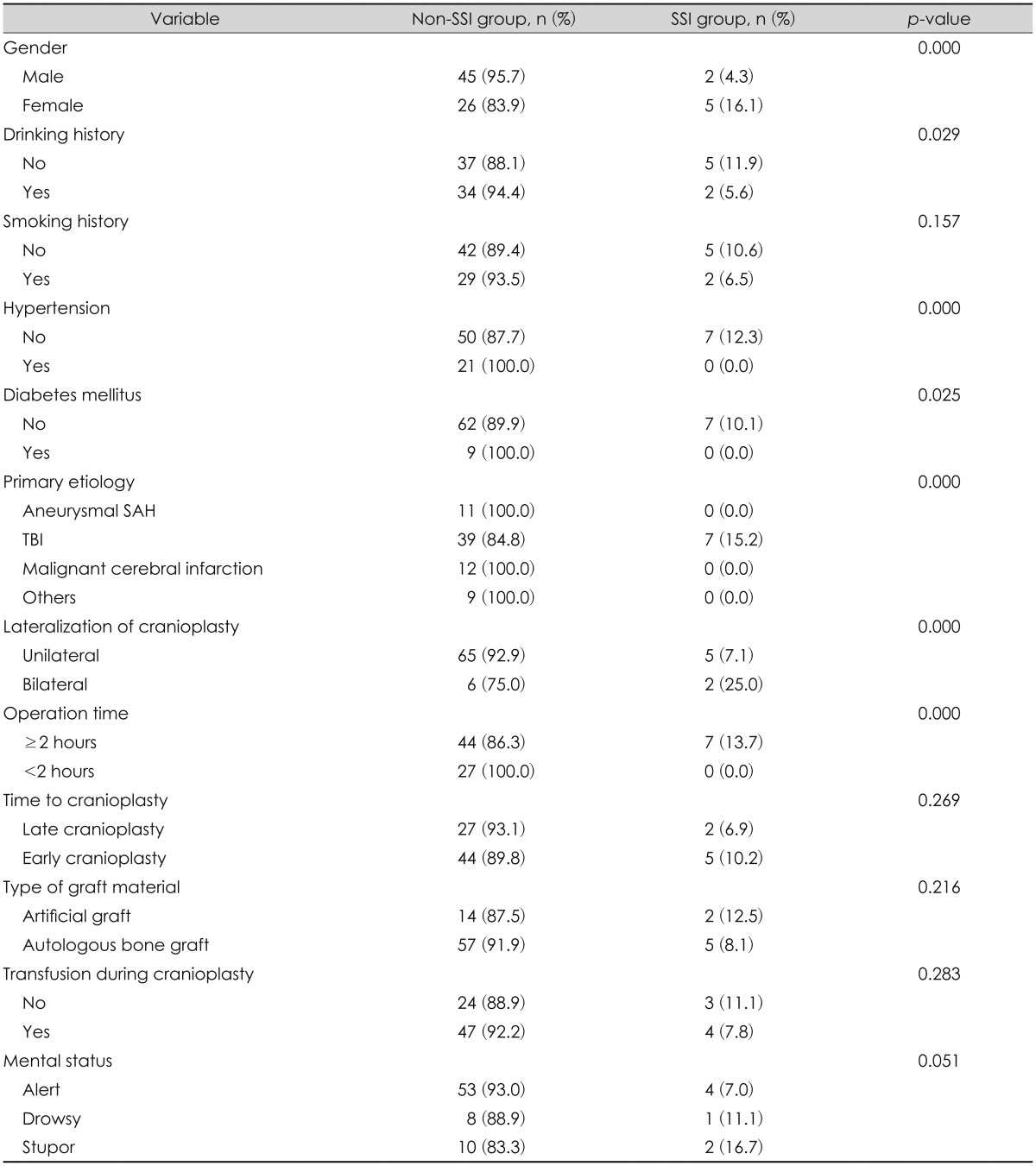
Among the 78 patients, SSIs after cranioplasty occurred in 7 (9.0%). The mean age of the SSI group was 43.0±18.1 years, which was significantly younger than that in the non-SSI group (53.9±14.93 years, p=0.046).
The differences in the variables between the two groups were observed in the patient-specific factors, such as gender, drinking history, HTN, DM, and primary etiology. The occurrence rate of SSIs among females was 4 times higher than it was among males (p=0.000). In the SSI group, no patients suffered any underlying comorbidities, such as HTN or DM. All patients in the SSI group had undergone decompressive craniectomy because of TBI. Among the surgery-specific factors, there were statistically significant differences in the SSI occurrence rate based on the lateralization of the cranioplasty and the operating time. The SSI rate in the group that had bilateral cranioplasty was significantly higher than that in the group whose surgery was only unilateral (25.0% vs. 7.1%, p=0.000). All operating times in the SSI group were greater than or equal to 2 hours. In terms of the time to cranioplasty or type of graft material, there were no differences in the SSI occurrence rate. The comparison of variables between the SSI and non-SSI groups is summarized in Table 2.
Univariate logistic regression analysis identified being female, having a primary etiology of TBI and having had a bilateral cranioplasty as having a significant association with developing SSIs after cranioplasty (Table 3). In the multivariate analysis, being female [odds ratio (OR) 5.98, p=0.000] and having had bilateral cranioplasty (OR 4.00, p=0.001) remained associated with a higher risk of infection.
Cranioplasty is associated with a relatively high overall complication rate ranging from 15 to 36.5%.5717181929) Furthermore, 25% to 76% of patients with post cranioplasty complications may need additional procedures to correct these complications.927) Thus, it is important to understand, prevent, and treat post cranioplasty complications. In particular, SSIs after cranioplasty have a tremendous impact on postoperative prognosis because they require surgical removal of the implanted graft material, prolonged antibiotic therapy and additional surgery for the cranioplasty. The SSI rate after cranioplasty ranges from 2% to 12%, according to the numerous studies published over the last 2 decades.5613141518) In the current study, the SSI rate was 9.0%, which is similar to those reported in the previous studies.
A number of studies reported that the SSI rate with cranioplasty following decompressive craniectomy in severe TBI was superior to that in non-TBI etiologies.61820) The SSI rates in these studies in the TBI groups ranged from 0% to 38.5%, and in the non-TBI groups, they ranged from 0% to 14.3%.61820) In the current study, the SSI rate in the TBI group was 15.2% (7/46), but there were no SSIs in the non-TBI group. Furthermore, Howard et al.12) reported that cerebrospinal fluid infection developed in 22.2% (4/18) of patients who had closed TBI in the period between the decompressive craniectomy and the cranioplasty. The primary etiology that leads to the decompressive craniectomy can be considered a cofactor in susceptibility to SSI after cranioplasty.61820)
The timing of surgery is one of the most controversial factors related to SSIs after cranioplasty.3) Some studies have shown that patients who had early cranioplasties (<3 months) had better functional outcomes, whereas others have reported the benefits of delaying the surgery.232125262731) Rish et al.21) reported that a shorter interval from decompressive craniectomy to cranioplasty was related to poor outcomes. In their report, the complication rate in the group that underwent cranioplasty within 6 months after decompressive craniectomy was 7.9%, which was higher than the rate in the group that underwent cranioplasty over 12 months later.21) Furthermore, Tokoro et al.26) reported that SSIs after cranioplasty were higher in the early cranioplasty group who underwent the surgery within 3 months. In the current study, although there was no association with developing SSIs in the multivariate logistic regression analysis, the SSI rate in the group with early cranioplasty was 10.2%, which was higher than the rate of 6.9% in the late cranioplasty group. The purported advantage of late cranioplasty may be related to avoiding operating on a potentially contaminated wound. It is difficult to prove this issue clinically, and our small number of patients in each group limits the strength of any conclusions to be made regarding the data.
The overall cranioplasty operating time can be considered a meaningful predictor of post cranioplasty SSI.6913151618) In a recent study, the mean cranioplasty operating time was 126.5 minutes, and there was a significant increase in the infection rate to >20%, when the surgery took longer than 200 minutes.16) In the current study, the operating times in the SSI group were less than 120 minutes, and the post cranioplasty SSI rate was significantly higher in patients who had operating times of greater than or equal to 120 minutes (0% vs. 13.7%, p=0.000). Based on these results, it is important that a skilled surgeon perform the cranioplasty in order to reduce operating time.
There are relatively few clinical studies on the risk factors associated with developing post cranioplasty SSIs.6151618) In the current study, multivariate logistic regression analysis showed that being female was the only patient-specific factor that was associated with a higher risk of infection. However, prospective cohort studies of patient-specific factors that are associated with developing SSIs after cranioplasty are needed to validate our results.
As with any retrospective study, this study has inherent biases and limitations. First, our sample size was relatively small, which may have caused a selection bias. Second, surgery-specific factors, such as operating time and time to cranioplasty after decompressive craniectomy, were not controlled in order to analyze the patient-specific factors related to post cranioplasty SSIs, and these could have been confounding factors. Nevertheless, the results of the current study may help to improve the clinical ability to predict post cranioplasty SSIs and provide a solid foundation for the design of future studies to address unanswered questions.
The results of this study suggest that surgery-specific factors, such as cranioplasty after TBI, bilateral cranioplasty and operating time of greater than or equal to 120 minutes can increase the SSI rate after cranioplasty. Thus, it is important to consider these surgery-specific factors in order to reduce SSIs after cranioplasty, especially in females.
References
1. Agner C, Dujovny M, Gaviria M. Neurocognitive assessment before and after cranioplasty. Acta Neurochir (Wien). 2002; 144:1033–1040. discussion 1040PMID: 12382131.

2. Archavlis E, Carvi Y Nievas M. The impact of timing of cranioplasty in patients with large cranial defects after decompressive hemicraniectomy. Acta Neurochir (Wien). 2012; 154:1055–1062. PMID: 22527574.

3. Beauchamp KM, Kashuk J, Moore EE, Bolles G, Rabb C, Seinfeld J, et al. Cranioplasty after postinjury decompressive craniectomy: is timing of the essence? J Trauma. 2010; 69:270–274. PMID: 20699735.

4. Bullock MR, Chesnut R, Ghajar J, Gordon D, Hartl R, Newell DW, et al. Surgical management of acute subdural hematomas. Neurosurgery. 2006; 58(3 Suppl):S16–S24. discussion Si-SivPMID: 16710968.

5. Chang V, Hartzfeld P, Langlois M, Mahmood A, Seyfried D. Outcomes of cranial repair after craniectomy. J Neurosurg. 2010; 112:1120–1124. PMID: 19612971.

6. Cheng YK, Weng HH, Yang JT, Lee MH, Wang TC, Chang CN. Factors affecting graft infection after cranioplasty. J Clin Neurosci. 2008; 15:1115–1119. PMID: 18656363.

7. Chibbaro S, Di Rocco F, Mirone G, Fricia M, Makiese O, Di Emidio P, et al. Decompressive craniectomy and early cranioplasty for the management of severe head injury: a prospective multicenter study on 147 patients. World Neurosurg. 2011; 75:558–562. PMID: 21600512.

8. Di Stefano C, Sturiale C, Trentini P, Bonora R, Rossi D, Cervigni G, et al. Unexpected neuropsychological improvement after cranioplasty: a case series study. Br J Neurosurg. 2012; 26:827–831. PMID: 22702390.

9. Gooch MR, Gin GE, Kenning TJ, German JW. Complications of cranioplasty following decompressive craniectomy: analysis of 62 cases. Neurosurg Focus. 2009; 26:E9. PMID: 19485722.

10. Honeybul S, Ho KM, Lind CR, Gillett GR. Validation of the CRASH model in the prediction of 18-month mortality and unfavorable outcome in severe traumatic brain injury requiring decompressive craniectomy. J Neurosurg. 2014; 120:1131–1137. PMID: 24605836.

11. Honeybul S, Janzen C, Kruger K, Ho KM. The impact of cranioplasty on neurological function. Br J Neurosurg. 2013; 27:636–641. PMID: 23883370.

12. Howard JL, Cipolle MD, Anderson M, Sabella V, Shollenberger D, Li PM, et al. Outcome after decompressive craniectomy for the treatment of severe traumatic brain injury. J Trauma. 2008; 65:380–385. discussion 385-386PMID: 18695475.

13. Huang YH, Yang TM, Lee TC, Chen WF, Yang KY. Acute autologous bone flap infection after cranioplasty for postinjury decompressive craniectomy. Injury. 2013; 44:44–47. PMID: 22154044.

14. Jho DH, Neckrysh S, Hardman J, Charbel FT, Amin-Hanjani S. Ethylene oxide gas sterilization: a simple technique for storing explanted skull bone. J Neurosurg. 2007; 107:440–445. PMID: 17695404.

15. Kim H, Sung SO, Kim SJ, Kim SR, Park IS, Jo KW. Analysis of the factors affecting graft infection after cranioplasty. Acta Neurochir (Wien). 2013; 155:2171–2176. PMID: 24043415.

16. Lee CH, Chung YS, Lee SH, Yang HJ, Son YJ. Analysis of the factors influencing bone graft infection after cranioplasty. J Trauma Acute Care Surg. 2012; 73:255–260. PMID: 22743392.

17. Lu Y, Hui G, Liu F, Wang Z, Tang Y, Gao S. Survival and regeneration of deep-freeze preserved autologous cranial bones after cranioplasty. Br J Neurosurg. 2012; 26:216–221. PMID: 22103564.

18. Matsuno A, Tanaka H, Iwamuro H, Takanashi S, Miyawaki S, Nakashima M, et al. Analyses of the factors influencing bone graft infection after delayed cranioplasty. Acta Neurochir (Wien). 2006; 148:535–540. discussion 540PMID: 16467959.

19. Morina A, Kelmendi F, Morina Q, Dragusha S, Ahmeti F, Morina D, et al. Cranioplasty with subcutaneously preserved autologous bone grafts in abdominal wall-Experience with 75 cases in a post-war country Kosova. Surg Neurol Int. 2011; 2:72. PMID: 21697987.

20. Nagayama K, Yoshikawa G, Somekawa K, Kohno M, Segawa H, Sano K, et al. [Cranioplasty using the patient's autogenous bone preserved by freezing--an examination of post-operative infection rates]. No Shinkei Geka. 2002; 30:165–169. PMID: 11857940.
21. Rish BL, Dillon JD, Meirowsky AM, Caveness WF, Mohr JP, Kistler JP, et al. Cranioplasty: a review of 1030 cases of penetrating head injury. Neurosurgery. 1979; 4:381–385. PMID: 111153.
22. Saade N, Veiga JC, Cannoni LF, Haddad L, Araújo JL. Evaluation of prognostic factors of decompressive craniectomy in the treatment of severe traumatic brain injury. Rev Col Bras Cir. 2014; 41:256–262. PMID: 25295986.

23. Simard JM, Sahuquillo J, Sheth KN, Kahle KT, Walcott BP. Managing malignant cerebral infarction. Curr Treat Options Neurol. 2011; 13:217–229. PMID: 21190097.

24. Smith ER, Carter BS, Ogilvy CS. Proposed use of prophylactic decompressive craniectomy in poor-grade aneurysmal subarachnoid hemorrhage patients presenting with associated large sylvian hematomas. Neurosurgery. 2002; 51:117–124. discussion 124PMID: 12182408.

25. Thavarajah D, De Lacy P, Hussien A, Sugar A. The minimum time for cranioplasty insertion from craniectomy is six months to reduce risk of infection--a case series of 82 patients. Br J Neurosurg. 2012; 26:78–80. PMID: 21973063.
26. Tokoro K, Chiba Y, Tsubone K. Late infection after cranioplasty--review of 14 cases. Neurol Med Chir (Tokyo). 1989; 29:196–201. PMID: 2477724.
27. Wachter D, Reineke K, Behm T, Rohde V. Cranioplasty after decompressive hemicraniectomy: underestimated surgery-associated complications? Clin Neurol Neurosurg. 2013; 115:1293–1297. PMID: 23273384.

28. Walcott BP, Kwon CS, Sheth SA, Fehnel CR, Koffie RM, Asaad WF, et al. Predictors of cranioplasty complications in stroke and trauma patients. J Neurosurg. 2013; 118:757–762. PMID: 23394335.

29. Wiggins A, Austerberry R, Morrison D, Ho KM, Honeybul S. Cranioplasty with custom-made titanium plates--14 years experience. Neurosurgery. 2013; 72:248–256. discussion 256PMID: 23149967.

30. Yadla S, Campbell PG, Chitale R, Maltenfort MG, Jabbour P, Sharan AD. Effect of early surgery, material, and method of flap preservation on cranioplasty infections: a systematic review. Neurosurgery. 2011; 68:1124–1129. discussion 1130PMID: 21242830.

31. Yamaura A, Sato M, Meguro K, Nakamura T, Uemura K. [Cranioplasty following decompressive craniectomy--analysis of 300 cases (author's transl)]. No Shinkei Geka. 1977; 5:345–353. PMID: 558536.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download