Abstract
Objective
Chronic subdural hematoma (CSDH) is a common intracranial hemorrhage, encountered in neurosurgical practice. Most CSDHs are unilateral, but some show bilateral involvement. However, the clinical characteristics of bilateral CSDH remain unclear. In this study, we investigated the clinical differences between bilateral and unilateral CSDH.
Methods
A retrospective study was performed on 120 patients with CSDH surgically treated at our institute from January 2008 to December 2012. Patients were divided into two groups: the bilateral CSDH and the unilateral CSDH groups. Clinical presentations, precipitating factors, computed tomography (CT) findings, postoperative complications, and outcomes of patients were analyzed.
Results
Bilateral CSDH was identified in 11 of 120 (10.9%) patients with CSDH. Patients with bilateral CSDH tended to have a lower rate of head injury compared to patients with unilateral CSDH (36.4% vs. 59.6%), but it had no statistical significance (p=0.201). The frequency of marked midline shift on CT scans was significantly greater in unilateral CSDH than in bilateral CSDH (p=0.010). Presenting symptoms, coexisting systemic diseases, postoperative complications, and clinical outcomes were not significantly different between the two groups.
Conclusion
Bilateral CSDH has comparatively similar clinical features and precipitating factors as unilateral CSDH. Patients with bilateral CSDH have significantly lower incidences of midline shift on CT scans, and most patients with either bilateral or unilateral CSDH have good postoperative outcomes.
Chronic subdural hematoma (CSDH) is one of the most common types of intracranial hemorrhages encountered in neurosurgical practice. Most CSDHs show unilateral lesions, but some show bilateral involvement. The overall incidence of bilateral CSDH varies from 14% to 25%.1,5,7,11,12,15) Compared to patients with unilateral CSDH, those with bilateral CSDH tend to be elderly (older than 75 years), have coagulation abnormalities, and experience a more aggressive clinical course.7,10,13) However, the clinical differences between bilateral and unilateral CSDH remain unclear.
The purpose of this study was to investigate the clinical features, clinical course, and postoperative outcomes for bilateral and unilateral CSDH. We compared with bilateral and unilateral CSDH and evaluated the factors influencing differences between the two patient groups.
A retrospective analysis was performed on 120 consecutive patients diagnosed with CSDH who were surgically treated at our institute during January 2008 to December 2012. CSDH diagnoses were confirmed by brain computed tomography (CT). Their medical records including clinical features, brain CT findings, surgical results, and postoperative status were reviewed.
All patients had undergone surgical intervention with burr hole and drainage. Under general anesthesia, one or two burr holes were trephined at the region of maximal hematoma. The subdural hematoma (SDH) was evacuated and/or washed out by irrigation with warm physiological saline solution. Closed system drainage of the SDH cavity using a soft silicon drain was performed in all cases for 1 to 3 days. For bilateral CSDH, burr holes were trephined on both sides of the head at the same time. Postoperative brain CT scans were obtained within 3 days following surgery. Subdural drainage catheters were removed following confirmation of near total removal of hematoma on postoperative brain CT scan and definite improvement of symptoms. After discharge from the hospital, brain CT scans were performed on a monthly basis until the SDH regressed and patients recovered to a premorbid functional status. Recurrence was defined as the occurrence of symptoms and signs attributable to an ipsilateral hematoma seen on a brain CT scan within 3 months of the first drainage procedure. Functional status on hospital admission was assessed using the Glasgow coma scale (GCS) and modified Rankin score (mRS).14,17) The Glasgow outcome scale and mRS were measured at discharge.6) All patients were followed postoperatively for more than 3 months.
Patients were divided into two groups: unilateral CSDH and bilateral CSDH. The following variables were compared between the two groups: sex, age, preceding head trauma, hypertension, diabetes mellitus, heart disease, cerebrovascular disease, liver disease, renal disease, antiplatelet therapy, anticoagulant therapy, seizure, presenting symptom (headache with nausea or vomiting, hemiparesis, aphasia, mental change, and seizure), preoperative functional status (mean GCS score, mean mRS score), and preoperative CT findings (degree of midline shift, hematoma thickness, and hematoma density).
Statistical analyses were conducted by performing Fisher's exact test and Student's t-test using the Statistical Package for the Social Sciences (SPSS) software (version 12.0; SPSS Inc., Chicago, IL, USA). In all analyses, a p-value of less than 0.05 was considered statistically significant.
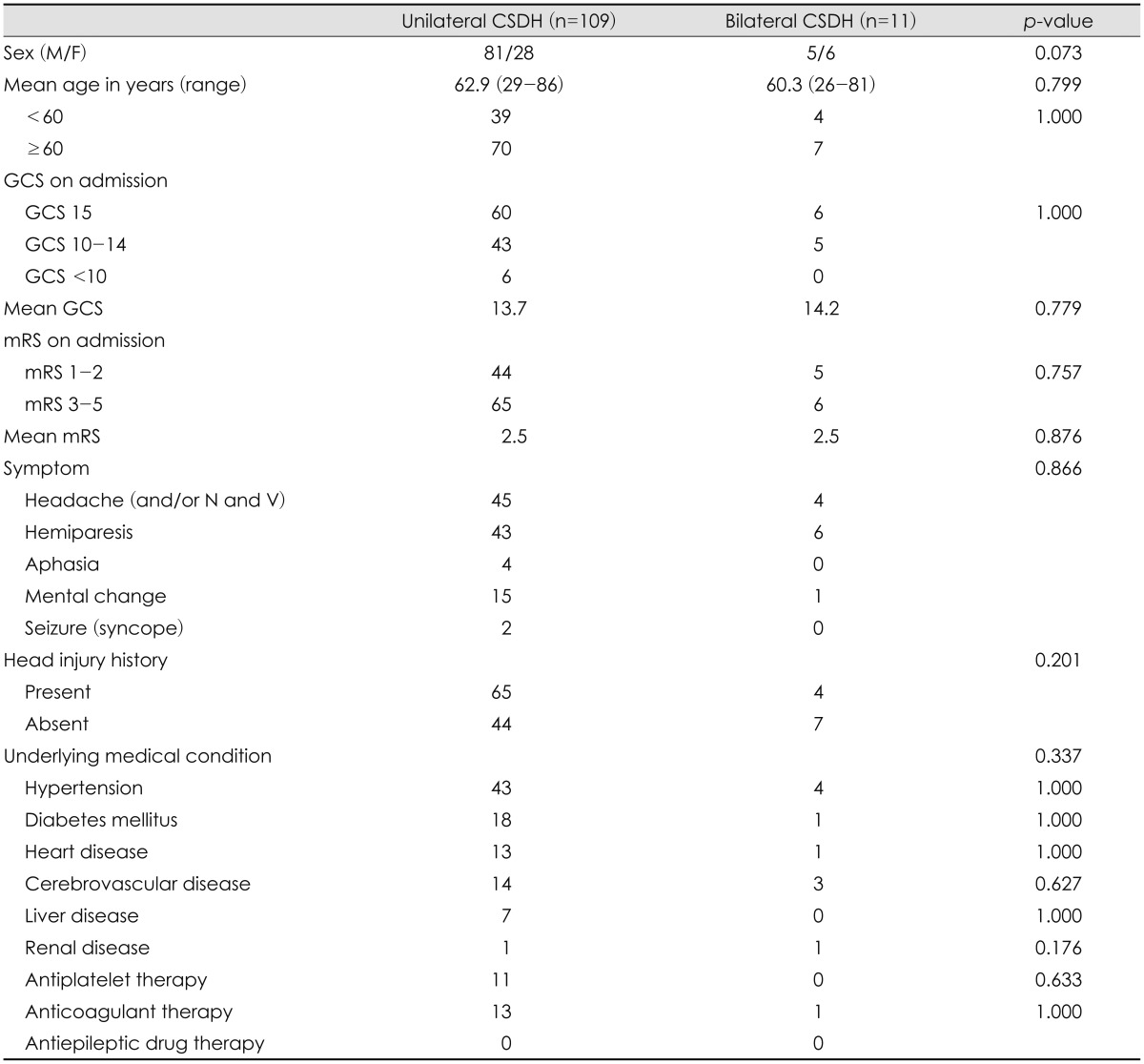
Patient demographic data and clinical characteristics are summarized in Table 1. The 120 patients (86 men and 34 wo-men) diagnosed with CSDH had a mean age of 62.7 years (range 26-86 years). With regard to the initial neurological status of the patients, the mean GCS score on hospital admission was 13.7 and the mean mRS score on admission was 2.5. Sixty-nine patients (57.5%) had a definite history of head injury. Hypertension was the most common underlying disease and was present in 47 patients (39.2%). Among the 120 patients, 11 were treated with bilateral trephination and drainage; in our patient sample, the incidence of bilateral CSDH was 10.9%.
We compared the clinical features of the unilateral and bilateral CSDH groups (Table 1). Differences in gender (p= 0.073), mean age (p=0.799), and advanced age group (≥60 years; p=1.000) were not statistically significant. The mean GCS on admission was 13.7 and 14.2 in the unilateral and bilateral CSDH patients, respectively (p=0.779). The mean mRS on admission was 2.5 and 2.5 in the unilateral and bilateral hematoma groups, respectively, and there was no significant difference between the groups (p=0.876). A history of head injury was found in 65 of 109 (59.6%) patients with unilateral CSDH and in four of 11 (36.4%) patients with bilateral CSDH. There were no statistically significant differences in the presentation of head injury history between unilateral and bilateral CSDH patients (p=0.201). The prevalence of underlying diseases, including hypertension, diabetes mellitus, heart disease, cerebrovascular disease, or others, was similar between the unilateral and bilateral CSDH groups. Statistical analyses revealed no significant intergroup differences in the main symptom of presentation (p= 0.337).
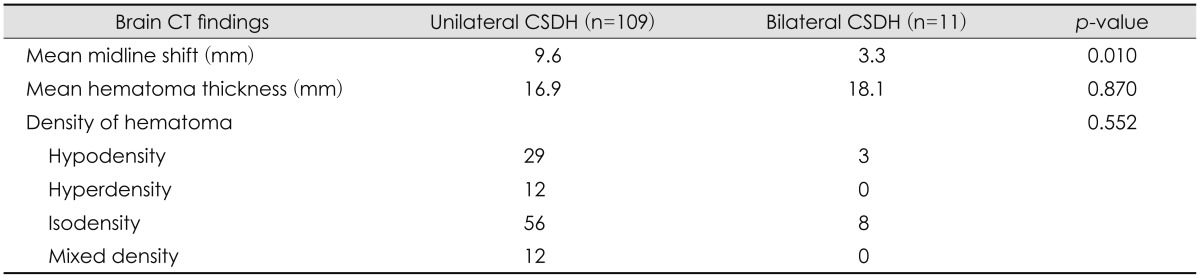
Preoperative radiological findings are compared in Table 2. The average degree of a midline shift on preoperative brain CT was 9.6 mm for unilateral CSDH and 3.3 mm for bilateral CSDH. Patients with unilateral CSDH had a significantly higher degree of midline shift on CT scans than those with bilateral CSDH (p=0.010). Hematomas thickness was 16.9 mm and 18.1 mm in patients with unilateral and bilateral hematoma, respectively (p=0.870), but hematoma density showed no significant differences between groups (p=0.552).
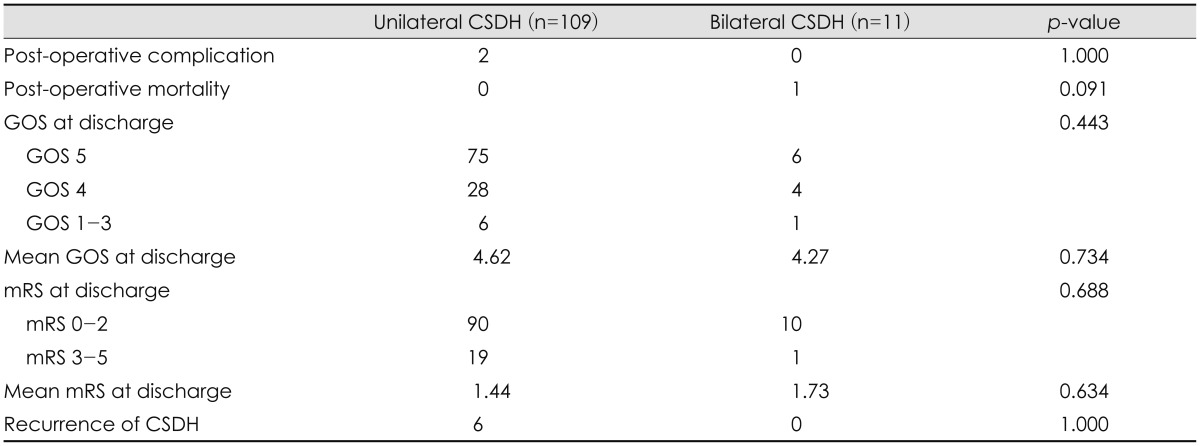
Postoperative complications and outcomes are shown in Table 3. There were two postoperative complications in our patients (1.6%), which included postoperative epidural hematoma and wound infection. Overall postoperative complications showed no significant differences between the groups. Most patients with CSDH had a good postoperative outcome after surgery, except for one patient who died (0.8%). The death was related to peritonitis caused by renal disease. Within 3 months after the operation, CSDH recurrence was observed in six patients (6/120, 5%). Thought the six recurrences of CSDH were found only in the unilateral CSDH group, there was no significant difference in recurrence between the groups (p=1.000).
CSDH is a fairly common neurosurgical disease, especially in the elderly population. Although recurrence is possible, postsurgical prognosis has been satisfactory.4) Bilateral CSDH is rare compared to unilateral CSDH, but was occasionally present in our clinical practice. The incidence of bilateral CSDH accounts for 14-25% of the cases of CSDH reported previously.1,5,7,11,12,15) Compared to unilateral CSDH, bilateral CSDH has an increased tendency for coagulopathy and has a more aggressive clinical course.7,16)
The incidence of bilateral CSDH in our patient group was 10.9%, which is slightly lower than the previously reported value. Although the pathophysiology of bilateral CSDH is not well understood, like unilateral CSDH, bilateral CSDH is postulated to be caused primarily due to traumatic injury to the bridging veins. Head trauma is an important precipitating factor for patients with CSDH. In our cohort, a history of head injury was present in 36.4% of patients with bilateral CSDH and 59.6% of patients with unilateral CSDH. These results show that bilateral CSDH has a lower rate of head injury, but there was no statistical difference between the groups. Several risk factors for bilateral CSDH have been reported; these include age (more than 75 years), coagulopathy, use of antiplatelet or anticoagulation medications, and hemodialysis.7,13) However, our analyses did not identify the abovementioned risk factors to predispose one to development of bilateral CSDH. In addition to the abovementioned factors, factors such as intracranial hypotension and skull shape have been identified as predisposing factors of bilateral CSDH in some studies.2,8) However, intracranial hypotension and skull shape were not evaluated in present study.
Clinical presentation of bilateral CSDH varies; there are no symptoms specific to patients with bilateral CSDH. It has been suggested that bilateral CSDH has an aggressive clinical course, and is affected by coagulofibrinolytic abnormalities, which somehow contribute to the enlargement of the SDH.7) It has also been suggested that bilateral CSDH has a lower incidence of hemiparesis compared to unilateral CSDH, possibly because there is less opportunity for the central brain structures to deviate owing to counterbalance of the mass effect on both hemispheres.5) This mass counterbalance effect is likely why patients with bilateral CSDH have a lower incidence of hemiparesis. In our patient group, the most common presentation was headache and hemiparesis in both bilateral and unilateral CSDH; yet, no significant difference was found between the groups.
We used brain CT for CSDH diagnosis and evaluated preoperative CT findings. Our CT data revealed no significant group differences in hematoma thickness and density. However, a marked midline shift in the preoperative CT was found to be significantly more frequent in unilateral CSDH than in bilateral CSDH (p=0.010). We estimated that the symptoms of our patient group reflect radiological findings. The unilateral CSDH group had an increased rate of midline shift compared to bilateral group, but the hematoma thickness was not different. Hematoma thickness in the bilateral CSDH group (mean, 18.1 mm) was larger than the unilateral CSDH group (mean, 16.9 mm), which might contribute to the lack of significant differences in clinical symptoms, especially hemiparesis, between our patient groups.
Most postoperative complications are related to procedural errors occurring during hematoma removal and subsequent drainage. In one recent meta-analysis of CSDH, the postoperative complication rate was estimated at 2.5-9.3%.3) In our study, two patients with unilateral CSDH experienced postoperative complications. One had an epidural hematoma and the other had a wound infection, requiring further treatment with surgery and antibiotics, respectively. Fortunately, the functional outcome at discharge of these two patients was not worse than their initial status. Finally, no significant difference was found between bilateral and unilateral CSDH groups regarding the postoperative complications we analyzed.
Most of our patients had a good postoperative outcome, consistent with previous reports.3,9) Previous work reports the mortality rate in CSDH varying between 2-12.2%.3,9) Because of the advanced age and multiple medical problems of patients, CSDH can be frequently associated with complications and mortalities. In our study, one patient (0.8%) died from peritonitis caused by renal disease.
Bilateral CSDH has been reported to be independent predictor for the recurrence of CSDH.5,12,15) Patients with bilateral CSDH tend to have previous brain atrophy, which may lead to poor brain re-expansion after surgery. Poor brain re-expansion correlates with recurrence and is thought to facilitate hematoma reaccumulation.9,12) In addition, some authors reported that bilateral CSDH occurs more frequently in patients with prolonged coagulation time.10) In our cohort, the six recurrences of CSDH were found only in the unilateral CSDH group, but the difference in the number of recurrences was not statistically significant (p=1.000).
Midline shift on CT scans was the only statistically significant difference between the bilateral and unilateral CSDH groups. This may arise due to limitations of this study, such as the retrospective nature of analysis, small sample size, and lack of existing research on contributing factors like intracranial hypotension, skull shape, and preoperative brain magnetic resonance imaging. We expect further analyses with more refined data could clarify the clinical significance of bilateral CSDH.
In our patient group, we showed that bilateral CSDH constituted 10.9% of CSDH, particularly in patients older than 60 years. Patients with bilateral CSDH had a lower rate of head injury history (36.4%), but we found no statistically significant difference in clinical features and precipitating factors between unilateral and bilateral CSDH. Compared with patients with unilateral CSDH, patients with bilateral CSDH had a significantly lower incidence of midline shift on CT scans. Most patients with bilateral or unilateral CSDH experienced good postoperative outcomes.
References
1. Chon KH, Lee JM, Koh EJ, Choi HY. Independent predictors for recurrence of chronic subdural hematoma. Acta Neurochir (Wien). 2012; 154:1541–1548. PMID: 22653496.

2. de Noronha RJ, Sharrack B, Hadjivassiliou M, Romanowski CA. Subdural haematoma: a potentially serious consequence of spontaneous intracranial hypotension. J Neurol Neurosurg Psychiatry. 2003; 74:752–755. PMID: 12754345.

3. Ducruet AF, Grobelny BT, Zacharia BE, Hickman ZL, DeRosa PL, Anderson K, et al. The surgical management of chronic subdural hematoma. Neurosurg Rev. 2012; 35:155–169. discussion 169. PMID: 21909694.

4. El-Kadi H, Miele VJ, Kaufman HH. Prognosis of chronic subdural hematomas. Neurosurg Clin N Am. 2000; 11:553–567. PMID: 10918029.

5. Huang YH, Yang KY, Lee TC, Liao CC. Bilateral chronic subdural hematoma: what is the clinical significance? Int J Surg. 2013; 11:544–548. PMID: 23707986.

6. Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975; 1:480–484. PMID: 46957.
7. Kurokawa Y, Ishizaki E, Inaba K. Bilateral chronic subdural hematoma cases showing rapid and progressive aggravation. Surg Neurol. 2005; 64:444–449. discussion 449. PMID: 16253697.

8. Lee KS. Natural history of chronic subdural haematoma. Brain Inj. 2004; 18:351–358. PMID: 14742149.
9. Mori K, Maeda M. Surgical treatment of chronic subdural hematoma in 500 consecutive cases: clinical characteristics, surgical outcome, complications, and recurrence rate. Neurol Med Chir (Tokyo). 2001; 41:371–381. PMID: 11561347.

10. Oyama H, Ikeda A, Inoue S, Shibuya M. [The relationship between coagulation time and bilateral occurrence in chronic subdural hematoma]. No To Shinkei. 1999; 51:325–330. PMID: 10363267.
11. Penchet G, Loiseau H, Castel JP. [Chronic bilateral subdural hematomas]. Neurochirurgie. 1998; 44:247–252. PMID: 9864695.
12. Robinson RG. Chronic subdural hematoma: surgical management in 133 patients. J Neurosurg. 1984; 61:263–268. PMID: 6737050.

13. Spallone A, Giuffrè R, Gagliardi FM, Vagnozzi R. Chronic subdural hematoma in extremely aged patients. Eur Neurol. 1989; 29:18–22. PMID: 2707288.

14. Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974; 2:81–84. PMID: 4136544.
15. Torihashi K, Sadamasa N, Yoshida K, Narumi O, Chin M, Yamagata S. Independent predictors for recurrence of chronic subdural hematoma: a review of 343 consecutive surgical cases. Neurosurgery. 2008; 63:1125–1129. discussion 1129. PMID: 19008766.
16. Tsai TH, Lieu AS, Hwang SL, Huang TY, Hwang YF.A comparative study of the patients with bilateral or unilateral chronic subdural hematoma: precipitating factors and postoperative outcomes. J Trauma. 2010; 68:571–575. PMID: 20065879.

17. van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988; 19:604–607. PMID: 3363593.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download