Abstract
Objective
Post-traumatic cerebral infarction (PTCI) is one of the most severe secondary insults after traumatic brain injury (TBI), and is known to be associated with poor outcome and high mortality rate. We assessed the practical incidence and risk factors for the development of PTCI.
Methods
We conducted retrospective study on 986 consecutive patients with TBI from the period May 2005 to November 2012 at our institution. The definition of PTCI was made on non-enhanced CT scan based on a well-demarcated or fairly discernible region of low attenuation following specific vascular territory with normal initial CT. Clinical and radiological findings that related to patients' outcome were reviewed and statistically compared.
Results
PTCI was observed in 21 (2.1%) patients. Of various parameters, age (p=0.037), initial Glasgow coma scale score (p<0.01), brain herniation (p=0.044), and decompressive craniectomy (p=0.012) were significantly higher in patients with PTCI than patients who do not have PTCI. Duration between accident and PTCI, patterns of TBI and vascular territory of PTCI were not specific. The mortality rates were significantly higher in patients with PTCI than without PTCI.
Post-traumatic cerebral infarction (PTCI) is one of the most severe secondary insult after traumatic brain injury (TBI), with a frequency ranging from 1.9% to 10.4%.12,19,21,22) It is occasionally associated with poor outcome and high mortality rate. Various mechanisms have been suggested for this complication, including direct vascular compression by mass effect, dissection, embolization, cerebral vasospasm, vascular injury, and systemic hypoperfusion.7,8,9,12,14,17,19,21,22) However, there were few clinical and radiological data defining early risk factors for development of PTCI. Early detection of patients who were at particularly high risk for PTCI would be very helpful in conducting management and predicting prognosis. We examined the practical incidence, and risk factors for the development of PTCI. By doing so, we can foretell the influence of PTCI on patient mortality.
We reviewed clinical and radiological data on 986 consecutive patients with TBI between May 2005 and November 2012 at our institution. All patients underwent initial computed tomography (CT) in the Emergency Department, and then they underwent subsequent CT surveillance during admission. We enrolled all clinical grade of patients who had any hemorrhagic findings on CT including skull fracture. The definition of PTCI was made on non-enhanced CT scan based on a well-demarcated or fairly discernible region of low attenuation following specific vascular territory.12) The admission CT revealed no abnormality and the subsequent low-density area is compatible with patients showing an acute onset of corresponding clinical signs and symptoms.
Patients with previous history of hypertension, cardiac disease, diabetes mellitus, hyperlipidemia or prior cerebral infarction were excluded lest we should include possible confounding factors. We analyzed also neurological status in terms of Glasgow coma scale (GCS) score, presence of brain herniation and duration from accident to onset of cerebral infarction.
The outcome at six months from the TBI was evaluated using the Glasgow outcome scale (GOS) as: good recovery (5), moderate disability (4), severe disability (3), vegetative state (2), and death (1). A good outcome was defined as a GOS score of 5 or 4, whereas a poor outcome was defined as a GOS score of 3, 2, or 1.
All patients were evaluated and treated according to the guidelines for the management of severe head injury.6) Neurological assessment was performed using the GCS score, pupil size and reaction.
For lowering intracranial pressure (ICP), patients received intravenous mannitol bolus infusion; at an initial dose of 0.5 g/kg, repeated at 4-8 hours, and guided by a target serum osmolality no more than 300-320 mOsm/L.
Patients with brain herniation syndrome and competent radiological findings of midline-shifting on CT scan undergo surgical decompression. Brain herniation syndromes were defined by both radiologic findings and neurological symptoms or signs. The pathognomonic imaging findings included displaced or effaced ventricles and cisterns, and the neurological symptoms or signs included progressive loss of consciousness, weakness of limbs, dilated pupils (>5 mm), loss of the pupillary reaction to light, and irregular breathing and pulse.
Decompressive craniectomy with a broad based, question mark, scalp flap centered on the superficial temporal artery was performed. The large bone flap (4 to 5 inches in its maximum diameter) was obtained on the side ipsilateral to the PTCI, and the temporal squama was removed until the floor of the middle cranial fossa was exposed. The dura was incised in cruciate fashion to allow for outward expansion of the brain, and dura was closed loosely with artificial materials. Brain tissues were not routinely removed, except from severe contusion. The bone has been stored under sterile conditions at -80℃.
Factors were expressed as the mean±standard deviations, and statistical analysis was carried out by using Statistical Package for the Social Sciences (SPSS) software for personal computers (SPSS ver. 19; SPSS Inc., Chicago, IL, USA). Chi-square test, Fisher's exact test was used for analysis of the independent contribution of predictive factors to the outcome. A backward stepwise elimination method was chosen for analysis to minimize omission of important variables. A probability value of less than 0.05 was considered as statistically significant.
Of the 986 patients evaluated, 21 (2.1%) developed cerebral infarctions during the three month study period. Table 1 shows the demographic and clinical data including initial GCS score, brain herniation, decompressive craniectomy and clinical outcome. Increasing age (≥65) was associated with PTCI (p=0.037), but sex does not meet statistical significance (p=0.495).
Mean duration between accident and the onset of infarction was 35.2 hours. Cerebral infarction appeared after injury within the first week in 7 (33.3%), 8 days to 2 weeks in 9 (42.8%), 2 to 3 weeks in 2 (9.5%), 3 weeks to 1 month in 2 (9.5%), and 1 to 3 months in 1 (4.8%). Because of small sample size and heterogeneous group, we could not verify a correlation between the ictus and latency of PTCI symptoms and ultimate outcome.
Traumatic brain lesions of CT findings were evaluated. In PTCI patients, the most common hemorrhage patterns were acute subdural hematoma (SDH) in ten patients (47.6%) and acute epidural hematoma in six patients (28.5%). Traumatic subarachnoid hemorrhage developed in three patients (18.2%) and traumatic intracerebral hematoma in one patient (4.7%). One patient (4.7%) had only skull fracture. This study revealed that any brain lesion could result in PTCI. However, acute SDH was the most common brain lesions.
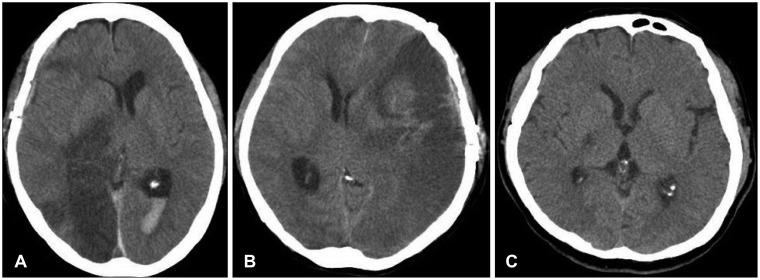
Sites of infarction territory were summarized in Table 2. Infarction was most common in the posterior cerebral artery (PCA) distribution in 8 patients (Figure 1A). Infarcts of the middle cerebral artery (MCA) territory were seen in five patients (Figure 1B). Anterior cerebral artery (ACA) and vertebrobasilar territory infarcts were diagnosed in two respectively. Anterior choroidal artery distribution was also seen 2 cases (Figure 1C), 1 in the ipsilateral side of main PTCI, while 1 in the opposite side of the PTCI.
Eleven patients (52.4%) were admitted to our institution with a GCS score of 3-6, 9 patients (42.9%) had a GCS score of 7-11, and only one (4.8%) had GCS scores over 12. The initial GCS score was an important factor for prediction of survival in our series (p<0.01). GCS results showed that 13 patients died, four patients were remained in a persistent vegetative state, one patient was severely disabled, two patients were moderately disabled and one patient showed good recovery at the time of six months from the TBI. The overall mortality rate was 61.9% (13 of 21 patients). The mortality rates were significantly higher in patients with PTCI than without, when matched for the initial GCS (Table 3). Of the eleven patients with a GCS score of less than 6, only two patients survived; of the 14 patients with a GCS score of 7 to 11, five patients survived. Especially, in patients with a GCS score of 7 to 11, mortality rate was higher in PTCI group than in no-PTCI group, and this difference was statistically significant (p<0.01).
The secondary effects of TBI might be more critical than the primary injuries. Brain edema, intracranial hemorrhage, vasospasm, and posttraumatic hydrocephalus are relatively common secondary brain injuries, and many studies have been conducted to explore their development following TBI.1,5,7,10,24) Cerebral infarction after brain trauma has also been recognized as a potential secondary injury, but only a few studies have reported the association between cerebral infarction and TBI. In this study, we tried to verify some factors responsible for the development of PTCI.
Of the 986 patients with TBI in this study, cerebral infarction was observed in 21 patients (2.1%) within 3 months after trauma. In one retrospective cohort study, the prevalence of cerebral infarction was 1.9%.12) Other studies revealed that prevalence of PTCI was up to 10.4%.19,21,22) These studies included only moderate or severe brain trauma patients. However, patients with TBI of all grades of severity were enrolled in this investigation. Therefore, our result showed low incidence of PTCI compared to other studies. When excluding patient with GCS score of 12 to 15, incidence of PTCI was 6.9%.
In most patients, PTCI developed early in the hospital course. In 16 (76.2%) patients, the infarction developed within 2 weeks after the injury. Therefore, we propose that CT or magnetic resonance diffusion studies are repeated within this period not to miss the diagnosis of PTCI. Cerebral infarction was rare, after two weeks from the initial TBI.
In this study, infarcts were seen most frequently in the area of the PCA, followed by the MCA. Infarcts in the small branch, such as anterior choroidal artery, lenticulostriate, and thalamoperforators were seen in four cases, respectively. These arteries have been found to be occluded against the skull base, resulting in infarction on basal ganglia after head traumas.2,4,12,13) In our study, infarction at the territory of PCA was the most common, consistent with the results of previous reports.12,21,22) Extra-axial hematoma can directly attribute to shifting or stretching of the MCA due to its mass effect or can indirectly affect MCA due to increased ICP, severe brain edema or herniation. And thus lead to MCA territory infarction.12,19,21)
PTCI can occur secondary to thrombo-embolic events from injured carotid or vertebral arteries. Vertebral artery dissection can occur in patients with head and neck trauma. In one series, 70% of vertebral artery dissections were accompanied by headache and 64% by vertebrobasilar ischemic symptoms.18) PTCI can also occur in a form of arterial vasospasm. One study by Pasqualin et al.14) reported incidence of posttraumatic angiographic vasospasm as 2% to 41%. But, due to lack of routine angiographic work up, our study has a limitation in figuring out factors affecting vertebral artery dissection in association with arterial vasospasm.
Our study suggested that increasing age (p=0.037), higher GCS at admission (p<0.01), brain herniation (p=0.044), and decompressive craniectomy (p=0.012) were risk factors for PTCI in patients with TBI. There was no statistical significance between cerebral infarction and sex (p=0.495) or abnormalities of laboratory findings.
Decompressive craniectomy has been frequently performed to control malignant brain edema by reducing ICP and resistance to cerebrospinal fluid outflow with decreased compression of cerebral vessels in cerebral infarction or trauma patients.11,20) It has long been suggested that decompressive craniectomy can improve survival rates and functional outcomes and decrease the occurrence of infarction in patients with severe brain edema when compared to medical treatment alone.3,23) But, inevitable bulging of brain tissue through the skull defect area in decompressive craniectomy can compress the brain tissue and vessels contained in the bulged area and thus can lead to cerebral infarction by inducing congestion, edema, ischemia and strangulation. Because decompressive craniectomy was performed when elevated ICPs threaten to cause lethal brain shifts, it may not simply be associated with PTCI as a risk factor. There was statistical significance between decompressive craniectomy and PTCI, however, further studies are needed to demonstrate these association.
Initial GCS score measuring post-resuscitative period was the most important factor for prediction of survival in this study. Decompressive craniectomy should be considered in patients with a GCS higher than 6, before increased ICP can contribute to an irreversible brain damage. On the other hand, the question of whether one should operate on a patient with a GCS of less than 6 or not, remains controversial. Careful selection of patients is important in order to avoid unnecessarily aggressive procedures in patients who have little chance of benefit or anticipated medical futility.
By the fact that PCA and MCA territory are the most frequently involved areas of the PTCI due to mechanical shifts, it can be suggested that posterior cerebral infarction (posterior temporal and occipital lobe) can be induced in medial temporal lobe herniation by the compression of the PCA against the rigid edge of the tentorium.17) Also, in case of ACA territory infarction, though the extent of infarction would vary by the degree of anteriorly-directed transfalcine herniation, it has been reported that the herniation of the hemisphere across the midline can cause the compression of the branches of the ipsilateral callosomarginal artery against the free edge of the falx.16)
The mortality rate in the patients of GCS 7 to 11 with PTCI in this study was 44.4%, significantly higher than in patients having TBI without PTCI (p<0.01), when matched by initial GCS. A somewhat higher mortality rate was found in PTCI group of GCS 3 to 6 and over 12, but the difference was not statistically significant (p=0.083, p=0.947). PTCI, as a complication in TBI, has been known to be an indicator of poor clinical outcome.12,15,22) Similarly, our result proved that PTCI is a direct manifestation of poor outcome. Therefore, aggressive treatment, including surgical procedure in patients with huge infarctions and refractory elevated ICP, as well as the administration of anticoagulants or calcium channel blockers, should be considered in patients with PTCI.
There are several limitations to this study. First, because this study was conducted at a single institution by a retrospective review of medical record and radiographic images, some discrepancies in the data interpretation and unexpected loss or omission of data might have been possible. To achieve external validity, multicenter prospective investigation should be required. Second, the subgroup analysis from a large population of different types of TBI patients is necessary in future because the small sample size and heterogeneity of the patient population.
In this study, PTCI occurred in 21 patients (2.1%). And PTCI results in high mortality rate and poor clinical outcome. This study suggested that increasing age, GCS at admission and brain herniation were risk factors for PTCI. Early recognition of these factors and prompt treatment may prevent PTCI, or at least minimize fatal consequences.
References
1. Czosnyka M, Copeman J, Czosnyka Z, McConnell R, Dickinson C, Pickard JD. Post-traumatic hydrocephalus: influence of craniectomy on the CSF circulation. J Neurol Neurosurg Psychiatry. 2000; 68:246–248. PMID: 10702038.

2. Dharker SR, Mittal RS, Bhargava N. Ischemic lesions in basal ganglia in children after minor head injury. Neurosurgery. 1993; 33:863–865. PMID: 8264884.

3. Eberle BM, Schnüriger B, Inaba K, Gruen JP, Demetriades D, Belzberg H. Decompressive craniectomy: surgical control of traumatic intracranial hypertension may improve outcome. Injury. 2010; 41:894–898. PMID: 21574279.

4. Endo M, Ichikawa F, Miyasaka Y, Yada K, Ohwada T. Capsular and thalamic infarction caused by tentorial herniation subsequent to head trauma. Neuroradiology. 1991; 33:296–299. PMID: 1922742.

5. Greene KA, Marciano FF, Johnson BA, Jacobowitz R, Spetzler RF, Harrington TR. Impact of traumatic subarachnoid hemorrhage on outcome in nonpenetrating head injury. Part I: A proposed computerized tomography grading scale. J Neurosurg. 1995; 83:445–452. PMID: 7666221.
6. Guidelines for the management of severe head injury. Brain Trauma Foundation, American Association of Neurological Surgeons, Joint Section on Neurotrauma and Critical Care. J Neurotrauma. 1996; 13:641–734. PMID: 8941879.
7. Han SR, Lee SJ, Yee GT, Choi CY, Sohn MJ, Lee CH. Post-traumatic middle cerebral artery dissection: a case report. J Korean Neurotraumatol Soc. 2009; 5:22–24.
8. Inamasu J, Ichikizaki K, Matsumoto S, Nakamura Y, Saito R, Horiguchi T, et al. Mild hypothermia for hemispheric cerebral infarction after evacuation of an acute subdural hematoma in an infant. Childs Nerv Syst. 2002; 18:175–178. PMID: 11981630.

9. Macpherson P, Graham DI. Arterial spasm and slowing of the cerebral circulation in the ischaemia of head injury. J Neurol Neurosurg Psychiatry. 1973; 36:1069–1072. PMID: 4772721.

10. Mazzini L, Campini R, Angelino E, Rognone F, Pastore I, Oliveri G. Posttraumatic hydrocephalus: a clinical, neuroradiologic, and neuropsychologic assessment of long-term outcome. Arch Phys Med Rehabil. 2003; 84:1637–1641. PMID: 14639563.
11. Messing-Jünger AM, Marzog J, Wöbker G, Sabel M, Bock WJ. Decompressive craniectomy in severe brain injury. Zentralbl Neurochir. 2003; 64:171–177. PMID: 14634882.

12. Mirvis SE, Wolf AL, Numaguchi Y, Corradino G, Joslyn JN. Post-traumatic cerebral infarction diagnosed by CT: prevalence, origin, and outcome. AJR Am J Roentgenol. 1990; 154:1293–1298. PMID: 2110744.

13. Okuno T, Takao T, Ito M, Konishi Y, Mikawa H, Nakano Y. Infarction of the internal capsule in children. J Comput Assist Tomogr. 1980; 4:770–774. PMID: 7217420.

14. Pasqualin A, Vivenza C, Rosta L, Licata C, Cavazzani P, Da Pian R. Cerebral vasospasm after head injury. Neurosurgery. 1984; 15:855–858. PMID: 6514159.

15. Robertson SC, Lennarson P, Hasan DM, Traynelis VC. Clinical course and surgical management of massive cerebral infarction. Neurosurgery. 2004; 55:55–61. discussion 61-62. PMID: 15214973.

16. Rothfus WE, Goldberg AL, Tabas JH, Deeb ZL. Callosomarginal infarction secondary to transfalcial herniation. AJNR Am J Neuroradiol. 1987; 8:1073–1076. PMID: 3120534.
17. Sato M, Tanaka S, Kohama A, Fujii C. Occipital lobe infarction caused by tentorial herniation. Neurosurgery. 1986; 18:300–305. PMID: 3703188.

18. Schievink WI, Mokri B, O'Fallon WM. Recurrent spontaneous cervical-artery dissection. N Engl J Med. 1994; 330:393–397. PMID: 8284004.

19. Server A, Dullerud R, Haakonsen M, Nakstad PH, Johnsen UL, Magnaes B. Post-traumatic cerebral infarction. Neuroimaging findings, etiology and outcome. Acta Radiol. 2001; 42:254–260. PMID: 11350282.

20. Shapiro K, Fried A, Takei F, Kohn I. Effect of the skull and dura on neural axis pressure-volume relationships and CSF hydrodynamics. J Neurosurg. 1985; 63:76–81. PMID: 4009278.

21. Tawil I, Stein DM, Mirvis SE, Scalea TM. Posttraumatic cerebral infarction: incidence, outcome, and risk factors. J Trauma. 2008; 64:849–853. PMID: 18404047.

22. Tian HL, Geng Z, Cui YH, Hu J, Xu T, Cao HL, et al. Risk factors for posttraumatic cerebral infarction in patients with moderate or severe head trauma. Neurosurg Rev. 2008; 31:431–436. discussion 436-437. PMID: 18704527.

23. Ziai WC, Port JD, Cowan JA, Garonzik IM, Bhardwaj A, Rigamonti D. Decompressive craniectomy for intractable cerebral edema: experience of a single center. J Neurosurg Anesthesiol. 2003; 15:25–32. PMID: 12499979.

24. Zubkov AY, Pilkington AS, Bernanke DH, Parent AD, Zhang J. Posttraumatic cerebral vasospasm: clinical and morphological presentations. J Neurotrauma. 1999; 16:763–770. PMID: 10521136.

FIGURE 1
Computerized tomography shows a post-traumatic cerebral infarction. A: Right posterior cerebral artery territory after ipsilateral epidural hematoma removal. B: Traumatic subarachnoid hemorrhage with post-traumatic cerebral infarction on the left middle cerebral artery territory. C: Post-traumatic cerebral infarction on the right anterior choroidal artery territory.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download