Abstract
Muscle relaxation using neuromuscular blocking agent is an essential process for endotracheal intubation and surgery, and requires adequate recovery of muscle function after surgery. Residual neuromuscular blockade is defined as an insufficient neuromuscular recovery that can be prevented by confirming train-of-four ratio >0.9 using objective neuromuscular monitoring. Sugammadex, a novel selective relaxant-binding agent, produces rapid and effective reversal of rocuronium-induced neuromuscular blockade. We report a case of the residual neuromuscular blockade accompanying dyspnea and stridor after general anesthesia in an unrecognized pre-existing symptomless unilateral vocal cord paralysis patient, who had experienced the disappearance of dyspnea and stridor after administration of sugammadex.
In terms of general anesthesia, the neuromuscular blocking agent is one of the most important factors that facilitates tracheal intubation for airway maintenance and helps to accommodate appropriate ventilation patterns of mechanical ventilation, as well as providing appropriate muscle relaxation for surgical manipulation [1]. Monitoring and management of muscle relaxation during general anesthesia can help safe recovery from residual neuromuscular blockade in the post-anesthesia care unit (PACU) and help reduce complications. Residual neuromuscular blockade is defined as an insufficient neuromuscular recovery that can be prevented by confirming train-of-four (TOF) ratio >0.9 using objective neuromuscular monitoring [12]. Extubation without sufficient reversal after neuromuscular block may cause complication such as dyspnea.
In this regard, acceleromyography can be used to monitor the degree of neuromuscular blockade, and γ-cyclodextrins, such as sugammadex (Org 25969; NV Organon, a part of Schering-Plough Corporation, Oss, The Netherlands), which can be used to reverse the neuromuscular block by binding directly to the neuromuscular blocking agent, have helped to recover the muscular strength of patient [3].
This report is to present a case of 82-year-old patient that had dyspnea due to residual neuromuscular blockade with unrecognized unilateral vocal cord paralysis, and dyspnea symptom improved through sugammadex administration with a review of the literature.
An 82-year-old man, who was 159 cm tall and weighed 47 kg, was planned to undergo transurethral resection of bladder as a bladder tumor under general anesthesia. The America Society of Anesthesiologists physical status classification was 2. The patient was taking amlodipine, a calcium channel blocker, after a diagnosis of hypertension 10 years ago. Six years ago, laparoscopic cholecystectomy was performed under general anesthesia by tracheal intubation using direct laryngoscopy. On the anesthesia records and recovery room records, operation time was 1 hour and the anesthesia time was 2 hours and 25 minutes without any specific findings. There were no abnormal findings in preoperative evaluation test such as chest X-ray, electrocardiogram, blood test, pulmonary function test, echocardiography. The patient arrived in the operating room without specific premedication. The patient was positioned with supine position. Electrocardiogram, noninvasive blood pressure monitor and peripheral oxygen saturation monitor was mounted, and the pre-anesthetic vital sign was blood pressure 180/80 mmHg, heart rate 75 beats/min, SpO2 99%. Before induction of general anesthesia, preoxygenation was done through spontaneous ventilation with 100% oxygen 6 L/min for 1 minute using mask. Patient received glycopyrrolate 0.2 mg, and midazolam 1 mg. The general anesthesia was induced with propofol 80 mg, fentanyl 100 µg, rocuronium 30 mg intravenously. The endotracheal intubation was done without difficulty using 7.5 mm cuffed endotracheal tube (tracheal tube; Mallinckrodt Medical, Athelone, Ireland) by direct laryngoscopy with no. 3 Macintosh blade after 90 seconds. With laryngoscopy, a laryngeal view score by Cormack and Lehane was two. The cuff of the endotracheal tube was filled with a minimum amount of air so that no gas leaks from the trachea, fixed at 23 cm from the gingiva of the incisors, and a breathing sound was confirmed in the whole lung field using a stethoscope.
The patient's position was changed with the lithotomy position. Anesthesia maintenance was performed by inhalation of nitrous oxide and oxygen at a total flow rate of 3 L/min with a ratio of 50:50, and the concentration of sevoflurane was adjusted to 1.0 to 2.0 vol% so that the vital sign was controlled within 20% of the preoperative blood pressure. During surgery, blood pressure was maintained at 120/75 to 150/80 mmHg, heart rate was 70 to 80 beats/min, peripheral oxygen saturation was maintained at 100%, and end-tidal CO2 was maintained at around 30 mmHg. Operation time was 25 minutes and fluid intake during surgery was 400 mL. The volume of bladder irrigation using saline was 9,000 mL and the amount of estimated blood loss was minimal.
After the end of surgery, the patient's position was changed from the lithotomy to the supine. By visual assessment using a peripheral nerve stimulator, four responses to TOF stimulation were observed in the adductor pollicis muscle. After that, all the anesthetic agent was discontinued and the patient started to awaken with 100% oxygen at 6 L/min. Glycopyrrolate 0.4 mg and pyridostigmine 10 mg were administered for neuromuscular blockade reversal. Fifteen minutes later, the patient breathed regularly of 16 to 18 times per minutes and opened his eyes to verbal command. The tidal volume was measured at about 250 to 300 mL by a spirometer, and partial pressure of end-tidal CO2 was 35 to 40 mmHg. He sustained hand-grip for five seconds to verbal command. So extubation was performed. Total anesthesia time was 1 hour. Then the patient was transferred to the PACU with oxygen through a facial mask, and instructed to take a deep breath.
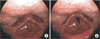
Peripheral oxygen saturation was maintained at 100% after transfer to the PACU. Five minute after the arrival at PACU, he complained of dyspnea with stridor and hoarseness, but his peripheral oxygen saturation was maintained at 100%. Manual positive pressure assisted ventilation was given via facial mask. He continued to complain of dyspnea, but the patient responded appropriately to instructions to hold his hand tightly with alert mental status. Even after administration of additional dose of reversal agent under the incomplete reversal of neuromuscular blockade, and dexamethasone 10 mg intravenous, his symptoms did not disappear. Sugammadex 100 mg was given after confirming TOF ratio of 0.68 on the acceleromyographic monitoring in abductor pollicis muscle by ulnar nerve stimulation. About 1 minute later, dyspnea and stridor disappeared and TOF ratio was 1.0. On portable flexible laryngoscopic examination by otolaryngologist, he had left vocal fold immobility and right vocal fold hypomobility which resulted very narrow vocal fold gap. After 40 minutes of sugammadex administration, the patient's condition remained stable except the hoarseness. Arterial blood gas analysis showed that PaCO2 was 46 mmHg, PaO2 was 200 mmHg, and oxygen saturation was 100% under 6 L/min of oxygen via facial mask. He was transferred to the general ward with 2 L/min of oxygen via nasal prong. His hoarseness persisted even after the transfer to the general ward, but the oxygen saturation was maintained more than 98%. On laryngoscopic re-examination after being transferred to general ward, his right vocal fold movement was improved with full abduction, but it was sluggish, and the left vocal fold still remained fixed at midline (Fig. 1). On his neck computed tomography on the day of operation, multiple thyroid nodules were found in both lobes. He was discharged on the next day of surgery. On the 11th postoperative day follow-up at otolaryngology clinic, it was confirmed that the left vocal cord paralysis persisted on laryngoscopy without any other complaints.
Incomplete recovery of neuromuscular blockade is one of the most common complications in the PACU [1]. Since the occurrence of residual neuromuscular blockade is affected by many clinical factors, it is difficult to know the incidence accurately. In general, residual neuromuscular blockade occurs because the effect of neuromuscular blocking agent is not completely eliminated after surgery. The anticholinesterase, which has a relatively short duration of action, increases the amount of acetylcholine in the neuromuscular junction in the early phase to compete with the neuromuscular blocking agent. However, when the concentration of anticholinesterase is decreased over time, residual neuromuscular blockade may occur due to the effect of the neuromuscular blocking agent remaining in the neuromuscular junction [4]. Risk factors contributing to residual neuromuscular blockade include age, gender, weight, renal or hepatic dysfunction, inhaled anesthetics, benzodiazepine, use of opioids, electrolyte imbalance, and hypothermia [4]. In addition, it has been reported that using a neuromuscular blocking agent at a high dose without neuromuscular monitoring may affect residual neuromuscular blockade [4].
Naguib et al. [5] reported that about 40% of patients undergoing general anesthesia with the use of neuromuscular blocking agent had TOF ratio of less than 0.9, and about 12% of patients in the PACU had TOF ratio of less than 0.7. Stewart et al. [1] also reported that TOF ratio was less than 0.9 in 31% of patients in the PACU. In addition, old age, laparotomy, and short operation time (<90 minutes) were risk factors for residual neuromuscular blockade [1]. In this patient, residual neuromuscular blockade effect may be considered because TOF ratio was 0.68 in the PACU. The patient was 82 years old, and the operation time was as short as 30 minutes, neuromuscular monitoring was not performed properly. Because of the clinical examinations with hand grip power, eye opening to the verbal command, and 250 to 300 mL of tidal volume with partial pressure of end tidal CO2 35 to 40 mmHg for respiration recovery without confirming TOF ratio, there is a possibility that the effect of inadequate neuromuscular block reversal in both time and dose has been achieved. In this case, the ability of head lift and masticatory force was not evaluated.
In the present case, we could think of the possibility that the unilateral vocal cord paralysis, which already existed, exacerbated the symptoms of dyspnea. The results of previous studies suggest that residual neuromuscular blockade may be seen in a many number of patients in the PACU, but in most cases, serious problems rarely occur [16]. However, the patients with reduced physiological reserve ability may have serious results [6]. In this case, the patient showed regular spontaneous breathing, opening of eyes according to the verbal command, and enough recovery of muscular strength after surgery, and extubation was performed. After the transfer to the PACU, the patient showed a stable appearance. However, the patient complained of dyspnea with stridor at the inspiration 5 minutes after arrival in the PACU. We suspected the upper airway stenosis and underwent flexible laryngoscopy to determine the cause of dyspnea. In the laryngoscopy, unilateral vocal cord paralysis with incomplete movement of the contralateral vocal cord was observed. Even if the unilateral vocal cord paralysis is present, dysphonia or swallowing difficulty may be absent because the movement of the contralateral vocal cord compensates the function of the paralyzed vocal cord [7]. In general, unilateral vocal cold paralysis is characterized by hoarseness, articulation disorder, mild dysphagia, or absence of symptoms, and symptoms tend to be alleviated after a certain period of time [8]. In this case, general anesthesia was performed for laparoscopic cholecystectomy 6 years ago, and it may be considered that unilateral vocal cold paralysis had occurred after that surgery. Rosenthal et al. [8] reported a retrospective study that 37 patients (5.8%) of 643 patients with unilateral vocal cord paralysis was related to endotracheal intubation. However, since it is difficult to conclude that the previous general anesthesia is the cause of vocal cold paralysis, neck computed tomography was performed after the surgery with the otolaryngologist consultation and it was confirmed that there were no other structural causes. As a result, the unilateral vocal cord paralysis has already been compensated by the function of the contralateral vocal cord, however, as the movement became imperfect due to the residual neuromuscular blockade, it is thought that airway obstruction may occur stridor and dyspnea symptom.
In order to prevent residual neuromuscular blockade, the dosage of neuromuscular blocking agent should be controlled by continuously monitoring the degree of neuromuscular block during general anesthesia using methods such as acceleromyographic measurement [9]. In addition to clinical examination such as hand-grip test and lifting the head for 5 seconds, it is possible to reduce the possibility of complication of residual neuromuscular blockade by performing an extubation after confirming the TOF ratio >0.9 [10].
In patients with respiratory dysfunction symptom, oxygen supplementation, assisted ventilation via a manual ventilator, and reintubation of the endotracheal tubes may be tried depending on the patient's condition, but in the end, it is best and important to reduce the effect of residual neuromuscular blockade [11]. When residual neuromuscular blockade occurs, additional administration of anticholinesterase may cause adverse effects such as nausea and vomiting, so it is possible to administer sugammadex. Sugammadex is selectively bound to rocuronium in plasma, resulting in a neuromuscular blocking agent that enters the plasma at a concentration difference in the neuromuscular junction and reduces the neuromuscular blocking effect [4]. The dosage should be based on the degree of neuromuscular blockade, 2 mg/kg when 2 single twitching in TOF, and 4 mg/kg when a deep block with post-tetanic counts of 1 or 2 in abductor pollicis muscle is administered [6]. If rocuronium is administered as much as the intubation dose, then 8 to 16 mg/kg of sugammadex is administered [6]. When appropriate doses are administered, the TOF ratio is recovered to 0.9 after 3 to 5 minutes [6].
We report a case of the patient with risk factors of residual neuromuscular blockade who had dyspnea symptom due to underlying diseases such as unrecognized unilateral vocal cord paralysis without neuromuscular monitoring. The patient had experienced a symptom improvement by administration of sugammadex. Therefore, appropriate neuromuscular monitoring should be performed whenever possible in all operations using neuromuscular blocking agent, and aggressive use of sugammadex in patients with risk factors may reduce residual neuromuscular blockade in the PACU. In addition, since unexpected underlying disease such as unilateral vocal cord paralysis may be present, it is important to be prepared to cope with residual neuromuscular blockade properly.
Figures and Tables
References
1. Stewart PA, Liang SS, Li QS, Huang ML, Bilgin AB, Kim D, et al. The impact of residual neuromuscular blockade, oversedation, and hypothermia on adverse respiratory events in a postanesthetic care unit: a prospective study of prevalence, predictors, and outcomes. Anesth Analg. 2016; 123:859–868.
2. Plaud B, Debaene B, Donati F, Marty J. Residual paralysis after emergence from anesthesia. Anesthesiology. 2010; 112:1013–1022.
3. Kim KS. Clinical use of sugammadex. Anesth Pain Med. 2011; 6:307–313.
4. Srivastava A, Hunter JM. Reversal of neuromuscular block. Br J Anaesth. 2009; 103:115–129.
5. Naguib M, Kopman AF, Ensor JE. Neuromuscular monitoring and postoperative residual curarisation: a meta-analysis. Br J Anaesth. 2007; 98:302–316.
6. Shin YS. Postoperative residual neuromuscular blockade. Anesth Pain Med. 2015; 10:1–5.
7. Mencke T, Echternach M, Kleinschmidt S, Lux P, Barth V, Plinkert PK, et al. Laryngeal morbidity and quality of tracheal intubation: a randomized controlled trial. Anesthesiology. 2003; 98:1049–1056.
8. Rosenthal LH, Benninger MS, Deeb RH. Vocal fold immobility: a longitudinal analysis of etiology over 20 years. Laryngoscope. 2007; 117:1864–1870.
9. Murphy GS, Szokol JW, Marymont JH, Greenberg SB, Avram MJ, Vender JS, et al. Intraoperative acceleromyographic monitoring reduces the risk of residual neuromuscular blockade and adverse respiratory events in the postanesthesia care unit. Anesthesiology. 2008; 109:389–398.
10. Eriksson LI. Evidence-based practice and neuromuscular monitoring: it's time for routine quantitative assessment. Anesthesiology. 2003; 98:1037–1039.
11. Kim DW, Kim BK, Kim JW, Kim JD, Ryu SJ, Kim DS. Late recurarization in the post-anesthetic care unit after total thyroidectomy: a case report. Anesth Pain Med. 2016; 11:380–383.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download