Abstract
Sarcoidosis is a multi-organ disease with various clinical manifestations. The lung is the most common site of manifestation; however, unusual findings may delay the correct diagnosis of sarcoidosis. Here we report a case of 32-year-old man with 4-month history of neck mass. Radiological findings revealed multiple pulmonary parenchymal nodules, with initial biopsy results of his neck lymph node showing chronic granuloma with focal necrosis. The patient was treated with anti-tuberculosis medications, but the size of the nodules did not change. Biopsy was performed from one of his pulmonary nodules, which revealed chronic granuloma without necrosis. Therefore, the patient was diagnosed with sarcoidosis. We present a case of sarcoidosis with multiple lung parenchymal nodules that is uncommon in Korea, with an aim to alert physicians of such unusual presentations.
Sarcoidosis is a multi-organ disorder of unknown etiology and presents various symptoms, such as fever, fatigue, malaise, and weight loss. It can also present in multiple organs, including lungs, skin, lymph nodes, liver, and bone marrow.
It is diagnosed through a combination of clinical presentation, radiological findings, and pathological confirmation. Lungs are frequently involved, and common radiological abnormalities include hilar and mediastinal lymph node enlargement and nodules along the bronchovascular bundles. However, sarcoidosis may present with various patterns on chest films; it is well known as the "the great mimic" [1]. Multiple lung nodules usually alert physicians to rule out metastatic lung disease; however, it is easy to neglect the possibility of pulmonary sarcoidosis under such circumstances. Multiple parenchymal nodular sarcoidosis is estimated to have prevalence of 2.4% to 4%, but is uncommon in Korea [23]. Here, we present an unusual case of sarcoidosis with multiple lung parenchymal nodules.
A 32-year-old previously healthy man with a history of smoking (15 pack-years, currently) presented with a 4-month history of a neck mass. Except for the neck mass, he did not experience any other discomfort, including fever, chills, cough, sputum, or joint pain. He had not traveled recently.
On physical examination, enlarged lymph nodes in both the supraclavicular area and the left elbow were palpable. His breathing sounds were normal, and he had no skin lesions. The findings from other clinical examinations were also unremarkable.
Complete blood cell count; electrolyte including calcium, creatinine, and blood urea nitrogen; results of coagulation and liver function tests; and C-reactive protein levels were all within normal limits. The carcinoembryonic antigen level was 2.0 ng/mL (normal range, 0 to 5 ng/mL); the angiotensin converting enzyme level 49.5 U/L (normal range, 8 to 52 U/L); the lactate dehydrogenase level 132 IU/L (normal range, 100 to 225 IU/L); and the squamous cell carcinoma antigen level 0.9 ng/mL (normal range, 0 to 2 ng/mL). Further, the neuron specific enolase level was 19.84 ng/mL (normal range, 0 to 16.3 ng/mL); and the cyfra 21-1 level was 5.64 ng/mL (normal range, 0 to 3.3 ng/mL), both of which were slightly elevated. The interferon gamma release assay yielded negative results, along with the sputum smear and the culture studies.
Simple chest films showed bilateral hilar bulging and parenchymal lung nodules (Fig. 1). A computed tomography (CT) scan of the chest revealed multiple lymph node enlargements in both side of the lower neck, the mediastinum, the axilla, and the abdomen, along with multiple well-defined nodules of variable size (range, 0.5 to 2.5 cm) in both lungs (Fig. 2).
Pulmonary function tests showed functional vital capacity (FVC) of 5.18 L (99%); forced expiratory volume in one second (FEV1) of 4.47 L (108%); FEV1/FVC of 86% after bronchodilator; and diffusing capacity for carbon monoxide of 85%. The results of electrocardiography were normal. Intimate evaluations of the central nervous system and the heart were not performed because of the absence of organ-specific symptoms.
A biopsy of his right neck lymph node revealed chronic granulomatous inflammation with focal necrosis. Considering such necrosis within a granuloma, as well as the high incidence rate of tuberculosis in young Korean populations, we diagnosed him with tuberculosis. The patient was initially treated with first-line tuberculosis medications (isoniazid 300 mg, rifampicin 600 mg, ethambutol 800 mg, and pyrazinamide 1,500 mg) for 2 months. However, the size of the neck lymph nodes did not change, and the results of a polymerase chain reaction tissue test were negative for Mycobacterium tuberculosis.
The patient stopped taking tuberculosis medications and underwent a percutaneous needle biopsy, of the lung nodule to rule out false negative results of tuberculosis lymphadenitis [45]. The biopsy revealed chronic granulomatous inflammation without necrosis surrounded by concentric layers of fibrosis, with no evidence of a microbiological organism (Fig. 3). Therefore, he was diagnosed with sarcoidosis.
The patient's lung nodules along with neck and elbow masses improved without any additional treatment (Fig. 2). He is currently monitored through regular visits to our outpatient department.
Lung involvement occurs in more than 90 % of patients with sarcoidosis; chest X-rays generally show bilateral hilar lymphadenopathy and pulmonary infiltrations. Because of such predominant lung involvement, a staging system has been developed dividing sarcoidosis into 5 phases: stage 0, no abnormalities; stage 1, lymphadenopathy; stage 2, lymphadenopathy and pulmonary infiltration; stage 3, pulmonary infiltration; and stage 4, fibrosis [1]. In this case, the patient's diagnosis was compatible with stage 2 sarcoidosis.
Multiple parenchymal nodules of the lung are one of the rare presentations of sarcoidosis [2]. When such parenchymal lesions appear in patients with advanced cancer, they may be confused with pulmonary metastasis [3]. Other than multiple nodular patterns, however, atypical lesions include airspace consolidation, ground-glass opacities, linear opacities, military opacities, and pleural thickening [16].
The diagnosis of sarcoidosis requires both adequate clinicoradiological findings and histological evidence [7]. It induces cascades of T-cell mediated immune reactions, which may either resolve on their own or result in the formation of a nonnecrotizing granuloma [28]. Sarcoidosis must be distinguished from other granuloma-forming diseases, such as tuberculosis, fungal infection, Blau syndrome, or granulomatous lesions of unknown significance [9]. In our case, the patient presented with multiple pulmonary nodules which is a rare manifestation for sarcoidosis [23]. Considering a disease presenting with lymph nodes enlargement with lung nodules, focal necrosis from biopsy tissue, and high prevalence of tuberculosis in young Korean populations, we could not completely rule out tuberculosis, which could worsen rapidly. Therefore, the patient was initially treated with anti-tuberculosis medications rather than simple observation over the 2-month follow-up period.
In addition, checking for systemic disease involvement is required, including ophthalmologic evaluation. The assessment of heart and central nervous system is required when clinically indicated [10]. The absence of an ophthalmologic evaluation and a gallium scan is a limitation of our case report. However, as also seen in our case, hilar adenopathy and pulmonary parenchymal presentation may narrow down the differential diagnosis into sarcoidosis, lymphoma, tuberculosis, or fungal infections [7].
In many cases, systemic treatment of sarcoidosis is not necessary [9]. The benefits of treatment should outweigh the risks of therapy when considering treatment; thus, the diagnosis should be definite [11]. The threshold for initiation of treatment for pulmonary sarcoidosis is not clearly defined, but most experts agree that chest film confirmed stages of 2 to 4, disease progression with overt symptoms, or loss of lung function over time (FVC decline 10% to 15%) warrants a treatment trial [712]. For patients with severe symptoms, treatment should be instituted at the time of diagnosis without an observation period. Glucocorticoids are the initial therapy of choice, but for patients that do not show improvement, further treatment with cytotoxic and anti-tumor necrosis factor agents should be considered [13]. Although the reported patient was compatible with stage 2 sarcoidosis that is an indication of treatment, we withheld treatment due to the absence of symptoms, presence of intact lung function, and no change of size over the two months.
The prognosis and natural course of sarcoidosis are highly variable. Most patients improve without any treatment, while 1% to 5% of patients die [14]. Usually, nodular lesions, airspace consolidations, and ground-glass opacities are considered reversible lesions, while honeycomb lesions, traction bronchiectasis, and architectural distortion are considered irreversible lesions [1].
In this article, we report a case of sarcoidosis that presented as multiple pulmonary nodules, which is uncommon in Korean populations. The recent impact on low dose chest CT may increase incidental detection of pulmonary parenchymal nodules, and such unusual presentations of sarcoidosis should be taken into account [15]. Clinical suspicion and early pathological confirmation should be sought for a correct diagnosis of sarcoidosis.
Figures and Tables
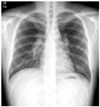 | Fig. 1Initial simple chest radiograph. It reveals hilar lymph node enlargement with multiple parenchymal lung nodules. |
References
1. Criado E, Sanchez M, Ramirez J, Arguis P, de Caralt TM, Perea RJ, et al. Pulmonary sarcoidosis: typical and atypical manifestations at high-resolution CT with pathologic correlation. Radiographics. 2010; 30:1567–1586.
2. Shahzad H, Ur-Rehman S, Fatima K, Sharif N, Zubairi AB. Case series and literature review of multiple nodular sarcoidosis. BMC Res Notes. 2013; 6:394.
3. Kim HS, Lee SY, Oh SC, Choi CW, Kim JS, Seo JH. Case report of pulmonary sarcoidosis suspected to be pulmonary metastasis in a patient with breast cancer. Cancer Res Treat. 2014; 46:317–321.
4. Pai M, Riley LW, Colford JM Jr. Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect Dis. 2004; 4:761–776.
5. Moon IS, Kim DW, Baek HJ. Ultrasound-based diagnosis for the cervical lymph nodes in a tuberculosis-endemic area. Laryngoscope. 2015; 125:1113–1117.
6. Park HJ, Jung JI, Chung MH, Song SW, Kim HL, Baik JH, et al. Typical and atypical manifestations of intrathoracic sarcoidosis. Korean J Radiol. 2009; 10:623–631.
7. Baughman RP, Culver DA, Judson MA. A concise review of pulmonary sarcoidosis. Am J Respir Crit Care Med. 2011; 183:573–581.
8. Kawar E, Saraf SY, Braman SS, Healey TT. Nodular pulmonary sarcoidosis. Med Health R I. 2012; 95:139–140.
9. Baughman RP, Lower EE, du Bois RM. Sarcoidosis. Lancet. 2003; 361:1111–1118.
10. Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007; 357:2153–2165.
11. Amin EN, Closser DR, Crouser ED. Current best practice in the management of pulmonary and systemic sarcoidosis. Ther Adv Respir Dis. 2014; 8:111–132.
12. Lazar CA, Culver DA. Treatment of sarcoidosis. Semin Respir Crit Care Med. 2010; 31:501–518.
13. Baughman RP, Nunes H, Sweiss NJ, Lower EE. Established and experimental medical therapy of pulmonary sarcoidosis. Eur Respir J. 2013; 41:1424–1438.
14. Hunninghake GW, Costabel U, Ando M, Baughman R, Cordier JF, du Bois R, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis. 1999; 16:149–173.
15. National Lung Screening Trial Research Team. Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011; 365:395–409.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download