Abstract
Myeloid sarcoma is a rare tumor mass consisting of immature granulocytic cells occurring in an extramedullary site or in a bone. It has often been observed during the course of an acute leukemia, myelodysplastic syndrome or myeloproliferative neoplasms, and it can involve any site of the body. However, it rarely present in the absence of bone marrow infiltration, especially for the isolated spinal myeloid sarcoma. In this report, we describe a case of isolated myeloid sarcoma that showed spinal compression. A 66-year-old male, with no underlying disease or medication history, presented with a progressive back pain and numbness in bilateral lower extremities that had begun two weeks before. He was diagnosed with myeloid sarcoma with no evidence of bone marrow involvement. Tumor cells were positive for CD34, c-KIT, and Bcl-2 on the immunohistochemical stain. He was treated with systemic chemotherapy with daunorubicin plus cytosine arabinoside and achieved a partial response.
Myeloid sarcoma, also called granulocytic sarcoma is a rare extramedullary tumor consisting of immature granulocytic cells [1]. It has often been reported in the course of an acute leukemia, myelodysplastic syndrome or myeloproliferative neoplasms including chronic myelogenous leukemia and essential thrombocythemia [1234]. Myeloid sarcoma can involve any site of body. However, spinal cord invasion without a known pre-existing or concomitant diagnosis of hematologic diseases is rare [13456]. Here, we present an unusual case of myeloid sarcoma that showed spinal compression without bone marrow involvement.
A previously healthy, 66-year-old male presented with a progressive back pain and numbness in bilateral lower extremities for two weeks. His past medical history was otherwise unremarkable. This patient had a normal mental status, with no evidence of central nervous system disorders. His power of both lower extremities were decreased to 4/5 sensory was also impaired in the level below the umbilicus. Rectal examination showed normal anal sphincter tone.
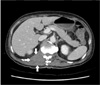
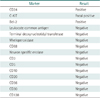
On admission, his complete blood count revealed no abnormal findings (Table 1). Computed tomography (CT) of the lumbosacral spine showed a diffuse infiltrative mass in the right paraspinal area (Fig. 1). The mass involved psoas, erector spinae, quadratus lumborum muscles, anterior longitudinal ligament and adjacent bone (T12–L2) with epidural involvement between T8 and L1 that resulted in compression of the spinal cord (Fig. 2). Histological sections from ultrasound-guided percutaneous core-needle biopsy of the tumor in psoas muscle showed diffuse infiltration of small round malignant cells with a few eosinophilic granular myeloblasts (Fig. 3). The malignant cells are positive for CD34, Bcl-2 and c-KIT by immunohistochemical stain (Fig. 4). Other immunophenotyping markers and Epstein-Barr virus were all negative. Ki-67 labeling index was up to 70 % (Table 2). The final pathological diagnosis of the tumor was myeloid sarcoma. The bone marrow biopsy and aspiration showed normocellular bone marrow with no evidence of leukemic cell infiltration (Fig. 5). Cytogenetic study of the bone marrow cells revealed a normal karyotype. Lumbar cerebrospinal fluid analysis was also negative for malignant cells.
On the 7th hospital day, he had a power of 2/5 in both the lower extremities with decreased sensations below L2. Rectal examination showed decreased anal sphincter tone.
He was immediately started on dexamethasone and local radiotherapy to the spinal axis for cord compression. Subsequently, he received induction chemotherapy with daunorubicin plus cytosine arabinoside and intrathecal treatment with methotrexate. After three weeks of treatment, computed tomography (CT) findings showed a partial response of the tumor (Fig. 6). However, his sensory and motor functions were not improved and further treatment was refused by the patient and his family.
Myeloid sarcoma occurs most often with hematologic malignancies and is reported in patients with acute myeloid leukemia, accelerated phase or blast crisis of chronic myelogenous leukemia with a range of 0.7%–9.1% [35]. Although any site of the body can be involved, the common locations are soft tissue, bone, periosteum, lymph nodes, peritoneum, and gastrointestinal system [134]. Clinical presentation of the disease is diverse with symptoms and signs determined by the size and localization of the tumor [13].
Isolated, primary or nonleukemic myeloid sarcoma, defined by the absence of hematologic malignancies, is uncommon [3]. Only a few cases of isolated myeloid sarcoma have been presented with spinal involvement and cause spinal cord compression or cauda equine syndrome [67]. It is estimated that 13% to 19% of patients with isolated myeloid sarcoma had central nervous system involvement such as head or spinal cord involvement [789]. The presenting symptoms of myeloid sarcoma causing spinal cord compression varied, including local pain, motor deficits, loss of bowel/bladder function, paraplegia, numbness and loss of sensation according to the size and location of the tumor [37]. Shiozawa et al. [7] have summarized that local pains were seen in 88% of patients having myeloid sarcoma causing cord compression, with 50% of these reporting back pain as in the present case.
Clinical diagnosis of isolated myeloid sarcoma is difficult because of its rarity and histological and radiological similarities to other malignant tumor. Many of myeloid sarcoma patients without bone marrow involvement were often misdiagnosed (25%–47%) as malignant lymphoproliferative disorders including lymphoma, thymoma, myeloma, eosinophilic sarcoma, extramedullary hematopoiesis, mucosa associated lymphoid tissue, Ewing sarcoma, and carcinoma [3910].
The diagnosis is performed by tissue biopsy or fine needle aspiration. Special immunohistochemical stain using blast-associated antigens like CD34, and myeloid- and monocytoid-associated antigens such as CD13, CD14, CD117 (c-KIT), and myeloperoxidase are needed to establish the exact diagnosis of myeloid sarcoma [3910]. In addition, flow cytometry, fluorescence in situ hybridization, and molecular analysis are helpful for the diagnosis.
There are few large randomized studies which examined the prognosis of isolated myeloid sarcoma. Immediate systemic chemotherapy with agents used in patients with acute myeloid leukemia is recommended to all patients having isolated myeloid sarcoma, although the optimal timing and type of treatment of isolated myeloid sarcoma are controversial [134]. Systemic chemotherapy for isolated myeloid sarcoma had 65% of complete remission and 20 months of median survival regardless of radiotherapy [8]. Patients who underwent chemotherapy had a significantly longer overall survival and event-free survival time compared to those who did not undergo chemotherapy [1011]. Local therapy without systemic treatment (either surgical excision or radiotherapy) is not an optimal option due to high rate of progression to acute myeloid leukemia within a few months after local treatment. However, radiotherapy could be used in patients who need a rapid relief of symptoms, or have an inadequate response to chemotherapy [13]. In cases of isolated myeloid sarcoma with cord compression, surgical decompression was performed mostly. Shiozawa et al. [7] have summarized that among the 26 cases, surgical decompression was done for 19 cases and among these 19 cases, there were neurologic improvements in 11 cases and no improvement in two cases. Only two cases were reported to show improvement in neurologic symptom by using radiotherapy and systemic chemotherapy without surgical decompression [7]. Therefore, if neurologic symptom is accompanied, then surgical decompression should be considered first and then if myeloid sarcoma is diagnosed, systemic chemotherapy should be followed. The median time of progression to acute myeloid leukemia in patients with isolated myeloid sarcoma ranges from 5 to 12 months [36910]. Patients with isolated myeloid sarcoma require close follow-up, since delayed or inadequately treated isolated myeloid sarcoma could progress to acute myeloid leukemia [310].
Figures and Tables
Fig. 1
Computed tomography on admission. It shows extension of right paraspinal mass (arrows) into epidural space.
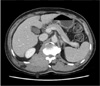
Fig. 2
Magnetic resonance (MR) image of the spine. (A) Axial T2-weighted MR image and (B) coronal T2-weighted fatsaturated MR image show epidural spinal cord compression (arrows).
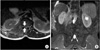
Fig. 3
Histologic finding of psoas muscle core-needle biopsy. (A) The sections show 4 cores of needle biopsied specimen (H&E, ×10). (B) High power field reveals small round blue cells infiltrating into skeletal muscle fibers. Some cells with eosinophilic granules (arrows) are also found, suggestive of immature blasts of eosinophil (H&E, ×400). (C) The tumor cells infiltrates into venous wall with partial disruption (H&E, ×200).
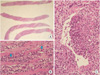
Fig. 4
Immunohistochemical staining for tumor. (A) CD34 is positive for tumor cells (×200). (B) Bcl-2 is positive for tumor cells (×200). (C) C-KIT is focal positive (×200). (D) The percentage of Ki-67 positive cells is 70% (×200).
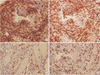
Fig. 5
Bone marrow aspirates and biopsy findings. (A) Hematopoietic cells of three lineages are within normal limit in number and distribution. No malignant cells are observed (Wright-Giemsa, ×400). (B) Bone marrow biopsy shows normocellular marrow. Hematopoietic cells of three lineages are within normal limit in number and distribution. No malignant cells are observed (H&E, ×100).

References
1. Yilmaz AF, Saydam G, Sahin F, Baran Y. Granulocytic sarcoma: a systematic review. Am J Blood Res. 2013; 3:265–270.
2. Desplechin A, Mekinian A, Rossignol J, Denis G, Gosset P, Cambier N, et al. Isolated megakaryoblastic bone sarcoma revealing acute myeloproliferative syndrome. Joint Bone Spine. 2010; 77:277–278.
3. Bakst RL, Tallman MS, Douer D, Yahalom J. How I treat extramedullary acute myeloid leukemia. Blood. 2011; 118:3785–3793.
4. Avni B, Koren-Michowitz M. Myeloid sarcoma: current approach and therapeutic options. Ther Adv Hematol. 2011; 2:309–316.
5. Chargari C, Jacob J, Bauduceau O, Ferrand FR, De Revel T, Vedrine L. Granulocytic sarcoma in a nonleukemic patient: place of radiotherapy and systemic therapies. Case Rep Med. 2011; 2011:929161.
6. Kalayci M, Sumer M, Yenidunya S, Ozdolap S, Acikgoz B. Spinal granulocytic sarcoma (chloroma) presenting as acute cord compression in a nonleukemic patient. Neurol India. 2005; 53:221–223.
7. Shiozawa Y, Kiyokawa N, Saito M, Fujimoto J, Hata J, Yamashiro Y. Granulocytic sarcoma of the spine in a child without bone marrow involvement: a case report and literature review. Eur J Pediatr. 2005; 164:616–620.
8. Tsimberidou AM, Kantarjian HM, Estey E, Cortes JE, Verstovsek S, Faderl S, et al. Outcome in patients with nonleukemic granulocytic sarcoma treated with chemotherapy with or without radiotherapy. Leukemia. 2003; 17:1100–1103.
9. Yamauchi K, Yasuda M. Comparison in treatments of nonleukemic granulocytic sarcoma: report of two cases and a review of 72 cases in the literature. Cancer. 2002; 94:1739–1746.
10. Antic D, Elezovic I, Milic N, Suvajdzic N, Vidovic A, Perunicic M, et al. Is there a "gold" standard treatment for patients with isolated myeloid sarcoma? Biomed Pharmacother. 2013; 67:72–77.
11. Imrie KR, Kovacs MJ, Selby D, Lipton J, Patterson BJ, Pantalony D, et al. Isolated chloroma: the effect of early antileukemic therapy. Ann Intern Med. 1995; 123:351–353.
12. Inoue T, Takahashi T, Shimizu H, Kanamori M, Kumabe T, Watanabe M, et al. Spinal granulocytic sarcoma manifesting as radiculopathy in a nonleukemic patient. Neurol Med Chir (Tokyo). 2008; 48:131–136.
13. Lee KH, Kim H, Choi GY, Lee JH, Han HS, Lee KH, et al. Granulocytic sarcoma presenting in two patients without leukemia. Korean J Med. 2009; 77:236–240.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download