Abstract
A 37-year-old male patient was admitted with generalized edema as the main symptom. A blood test confirmed hypoalbuminemia and hyperlipidemia, and a urine test confirmed severe albuminuria. A renal biopsy was conducted, which revealed a diagnosis of minimal change disease. Although the patient experienced complete remission of minimal change nephrotic syndrome after oral prednisolone and cyclophosphamide treatment, he is readmitted due to bilateral leg edema 5 years later since minimal change nephrotic syndrome was completely cured. The patient is diagnosed with IgA nephropathy. Although the exact mechanisms of IgA nephropathy in this patient remain unclear, this case represents an extremely rare development, and is separate from the remission of minimal change nephrotic syndrome.
Nephrotic syndrome is a clinical syndrome that includes the symptoms of albuminuria, hypoalbuminemia, hyperlipidemia, and generalized edema, and is categorized into an idiopathic syndrome with unknown causes and a secondary syndrome, which is caused by various systemic diseases [1]. In Korea, minimal change nephrotic syndrome is the most common cause for primary nephrotic syndrome in adults and is relatively responsive to steroid treatment [234]. After complete remission, there is about a 31%–56% rate of recurrence, which occurs mostly during the first year; recurrence more than 5 years after remission is very rare [56789]. There has been one domestic case where minimal change nephrotic syndrome recurred 15 years after complete remission [9]. No previous domestic cases have described minimal change nephrotic syndrome recurring as IgA nephropathy 5 years after remission.
A 37-year-old male patient who was diagnosed with and experienced complete remission of minimal change nephrotic syndrome 5 years prior is readmitted to the hospital with edema in both legs. After initial diagnosis with minimal change nephrotic syndrome, the patient was administered oral prednisolone for 2 years and cyclophosphamide for 3 months, and was observed on an outpatient basis after complete remission of minimal change nephrotic syndrome. Upon recent readmission, chest examination reveals that the patient’s lung sounds are normal, and abdominal palpation shows no signs of splenomegaly, hepatomegaly, or ascites. The patient has pitting edema in both legs.
Peripheral blood smear examination results show a WBC count of 6,810/mm3, a hemoglobin of 13.5 g/dL, and a platelet count of 215,000/µL. The biochemical examination results show a total protein of 4.3 g/dL, an albumin of 2.2 g/dL, a total cholesterol of 334 mg/dL, a triglyceride level of 115 mg/dL, a sodium of 133 mEq/L, a potassium of 4.3 mEq/L, a blood urea nitrogen concentration of 20 mg/dL, and a creatinine of 1.28 mg/dL. The urine test reveals a urine specific gravity of 1.020, a pH of 5.0, urinary protein 4+, 2–4 WBCs/HPF, and 0–2 RBCs/HPF, and the 24-hour urine test demonstrates a protein content of 11,924 mg/day. In addition, serological examination results include HBsAg (-), HBsAb (+), anti-HCV Ab (-), anti-HIV (-), a CRP of 0.3 mg/dL, and negative rheumatoid factor. C3 is 103 mg/dL (normal, 80 to 160 mg/dL), C4 is 27 mg/dL (normal, 10 to 40 mg/dL), the antistreptolysin O titer is 30 IU/mL (normal, 0 to 200 IU/dL), and IgA is 232 mg/dL (normal, 70 to 400 mg/dL).
When minimal change nephrotic syndrome was first diagnosed, segmental sclerosis with increased dense eosinophilic mesangial matrix was observed by light microscopy (LM) on tissue examination of the kidneys (Fig. 1), but electron-dense deposits were not observed on electron microscopy (EM) (Fig. 2). Complements and immune deposits were not observed by immunofluorescence (IF). In the tissue analysis conducted after recurrence, mesangial expansion with focal hypercellularity is observed by LM (Fig. 1), and electron-dense deposits at the mesangium with slight mesangial proliferation are observed on EM, but foot process effacement is not observed (Fig. 2).
Electron-dense deposits of the IgA immune complex including IgM are observed on IF (Fig. 3). Based on the results of the renal biopsy and the diagnosis of IgA nephropathy, a combination of oral cyclophosphamide (1.5 mg/kg/day) and oral prednisolone (40 mg per day tapered to 10 mg/day over two years) was administered for 4 months as the initial treatment, and albuminuria was partially improved. Later, the administration of oral steroids was tapered, and 1.5 mg/kg/day azathioprine was given at the recommended dosage for 28 months. Albuminuria and edema completely disappeared, and currently the patient is stable with no symptoms of recurrence.
Minimal change nephrotic syndrome comprises 25%–30% of primary nephrotic syndrome cases in Korean adults and is reported as the most common disease [12]. Some patients recover naturally, but most patients respond favorably to steroid treatment; 50% of adults show a positive response to steroid treatment. The recurrence rate in adults is lower than that in children, and about 31%–56% of adult patients show recurrence after complete remission. Cases of recurrence occur mostly within 1 year of remission, and it is rare to see patients with recurrence 5 years after complete remission. It is hypothesized that T-cells are instrumental in the pathogenesis of minimal change disease (MCD), as they release a cytokine called permeability factor that injures the glomerular epithelial foot processes [10]. This damage may lead to albuminuria in MCD patients. IgA nephropathy patients exhibit electron-dense mesangial deposits that stain positive for IgA [1112]. Recent studies have found that T-cell deposition in renal tubules in IgA nephropathy may be a marker of progression and severity. However, the role of T-cell activation and dysfunction in IgA nephropathy remains unclear [13]. Some patients with IgA nephropathy have acute-onset nephrotic syndrome, in which there is only mild mesangial proliferation or diffuse fusion of foot processes on renal biopsy, similar to that seen in MCD. Furthermore, many of these patients behave as if they have MCD, with remission of proteinuria induced by glucocorticoid therapy. Although the exact pathological mechanisms in this patient remain unclear, this case represents an extremely rare development, and secondary biopsy did not reveal foot process fusion, so it is separate from the remission of minimal change nephrotic syndrome. Based on this case, adults with minimal change nephrotic syndrome require continuous monitoring even after complete remission, and there is a need for additional research on the mechanisms of recurrence after remission.
Figures and Tables
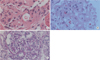 | Fig. 1Renal biopsy (H&E, ×400). (A) The H&E stained section (first biopsy) shows segmental sclerosis with increased dense eosinophilic mesangial matrix. (B) The second H&E stained section (second biopsy) demonstrates mesangial expansion with foamy cell collection. (C) The last periodic acid-Schiff (PAS) stained slide shows slight mesangial proliferation. |
References
1. Kikuchi M, Yumura W, Sugino N. Definition and classification of nephrotic syndrome. Nihon Rinsho. 1984; 42:1346–1351.
2. Sung JH, Hwang EA, Jin KB, Kwak JH, Han SY, Park SB, et al. Clinical pathologic study on adults idiophathic nephritic syndrome in Korea. Korean J Nephrol. 2007; 26:61–69.
3. Nolasco F, Cameron JS, Heywood EF, Hicks J, Ogg C, Williams DG. Adult-onset minimal change nephrotic syndrome: a longterm follow-up. Kidney Int. 1986; 29:1215–1223.
4. Shin YT, Oh SM, Na KR, Kim JH, Hwang PJ, Kim JS, et al. Clinical characteristics of minimal change nephrotic syndrome in adults. Korean J Nephrol. 1998; 17:46–52.
5. Fujimoto S, Yamamoto Y, Hisanaga S, Morita S, Eto T, Tanaka K. Minimal change nephrotic syndrome in adults: response to corticosteroid therapy and frequency of relapse. Am J Kidney Dis. 1991; 17:687–692.
6. Chang KJ, Park SB, Kim HC. Long-term follow-up of adultonset minimal change nephrotic syndrome. Korean J Nephrol. 2003; 22:185–194.
7. Kida H, Iida H, Dohi K, Nakamoto Y, Mizumura Y, Takeuchi J. Period of freedom from relapse as an indication of cure in minimal change nephrotic syndrome in adults. Nephron. 1977; 19:153–157.
8. Kim YS, Suh WK, Lee TW, Ihm CG, Kim MJ. Clinical characteristics and therapeutic response of adult-onset minimal change disease. Korean J Nephrol. 1987; 6:276–282.
9. Shin DH, Kim JH, Kim JY, Noh YJ, Moon SY, Kim JG, et al. A case of adult-onset minimal change nephrotic syndrome relapsed after 15-year of complete remission. Korean J Nephrol. 2003; 22:608–611.
10. Cho MH, Hong EH, Lee TH, Ko CW. Pathophysiology of minimal change nephrotic syndrome and focal segmental glomerulosclerosis. Nephrology (Carlton). 2007; 12:Suppl 3. S11–S14.
11. Barratt J, Feehally J. IgA nephropathy. J Am Soc Nephrol. 2005; 16:2088–2097.
12. Donadio JV, Grande JP. IgA nephropathy. N Engl J Med. 2002; 347:738–748.
13. Oberweis BS, Mattoo A, Wu M, Goldfarb DS. Minimal change disease and IgA deposition: separate entities or common pathophysiology? Case Rep Nephrol. 2013; 2013:268401.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download