Abstract
Objectives
Methods
Results
Figures and Tables
Fig. 1
Hepatocellular carcinomas with lipiodol uptake on precontrast computed tomography with bone window setting. (A) The hepatocellular carcinoma (arrow) shows dense uptake of lipiodol in more than 90% of the area of the lesion in hepatic segment 6 and is classified into group A. (B) The hepatocellular carcinoma (arrow) reveals partial uptake of lipiodol in 50% to 90% of the area of the lesion in hepatic segment 5 and is classified into group B.
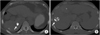
Fig. 2
MR images of a 69-year-old patient with a 1.9×1.3 cm-sized dense hepatocellular carcinoma with lipiodol uptake in hepatic segment 5. Hepatocellular carcinoma (arrows) shows dense uptake of lipiodol on precontrast computed tomography with bone window setting (A), high signal intensity on T2-weighted images (B), and low signal intensity on T1-weighted images (C). On diffusion-weighted images, it reveals high signal intensity at lower b factors (b=0 and b=50) (D and E, respectively) and decreased signal intensity at a higher b factor (b=800) (F). The lesion shows high signal intensity on in-phase gradient-echo images (G) and low signal intensity on out-of-phase gradient-echo images (H). It is not enhanced on dynamic contrast-enhanced arterial phase (I), portal phase (J) or 10-minute delayed phase (K) images.
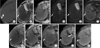
Fig. 3
MR images of a 57-year-old patient with a 2.3×2.3 cm-sized hepatocellular carcinoma with partial lipiodol uptake in hepatic segment 6. Hepatocellular carcinoma (arrows) shows partial uptake of lipiodol on precontrast computed tomography with bone window setting (A), high signal intensity on T2-weighted images (B), and low signal intensity on T1-weighted images (C). On diffusion-weighted images, the lesion reveals high signal intensity at a lower b factor (b=0) (D) and decreased signal intensity at a higher b factor (b=800) (E) images. It shows low signal intensity on in-phase (F) and out-of-phase (G) gradient-echo images. Hepatocellular carcinomas is not enhanced on dynamic contrast-enhanced arterial phase (H), portal phase (I) or 20-minute delayed phase (J) images.
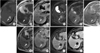
Fig. 4
MR images of a 73-year-old patient with a 0.7×0.6 cm-sized, small, hepatocellular carcinomas with dense lipiodol uptake in the hepatic segment 5. Hepatocellular carcinoma (arrows) shows dense uptake of lipiodol on precontrast computed tomography with bone window setting (A), low signal intensity on T2-weighted images (B) and T1-weighted images (C). Diffusion-weighted images reveals high signal intensity at a lower b factor (b=0) (D) and decreased signal intensity at a higher b factor (b=800) (E). Hepatocellular carcinoma with dense lipiodol uptake shows isointensity on in-phase gradient-echo images (F) and low signal intensity on out-of-phase gradient-echo images (G).

Table 1
Variations in signal intensity of HCCs with lipiodol uptake on MRI according to the amount of lipiodol uptake

Group A was consisted of HCCs with dense lipiodol uptake (more than 90%) and group B with partial lipiodol uptake (between 50% and 90%). One HCC in group A had no T2WI, and one HCC in group B had no diffusion weighted images (b=0).
HCC, hepatocellular carcinoma; T2WI, T2-weighted images; T1WI, T1-weighted images; DWI, diffusion-weighted images; GE in, in-phase gradient-echo images; GE out, out-of-phase gradient-echo images.
Table 2
Variations in signal intensity of HCCs with lipiodol uptake on MRI according to size

Group I was defined by a longest diameter of less than 2 cm, and group II was defined by a longest diameter of greater than or equal to 2 cm. One HCC in group II had no T2-WI, and another HCC in group II had no diffusion weighted images with b=0 value.
HCC, hepatocellular carcinoma; T2WI, T2-weighted images; T1WI, T1-weighted images; DWI, diffusion-weighted images; GE in, in-phase gradient-echo images; GE out, out-of-phase gradient-echo images.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download