Introduction
Influenza virus infection is a common respiratory disease in children that rarely results in renal complications, although acute kidney injury (AKI) occurred frequently in severely ill patients during the 2009 H1N1 influenza A virus pandemic [1234]. Also, AKI due to influenza B virus infection remains uncommon [56]. Kawasaki disease (KD) occurs infrequently associated with influenza A virus H1N1 infection [7], but it has not been reported with influenza B, even during the recent epidemics [6]. Recently, an epidemiologic study has documented the rare association of KD with influenza A and B virus [8]. Although renal complications of KD occur occasionally [91011], a simultaneous KD and AKI concomitant with an influenza virus infection have not been reported in the literature. Here, we report a 13-year-old girl with an influenza B virus infection who presented with AKI and was subsequently diagnosed with atypical KD. This is the first case of atypical KD with AKI occurring in association with an influenza B virus infection.
Case
A 13-year-old girl was admitted to the hospital due to anuria for 24 hours, which developed after a 3-day history of high fever, cough, coryza, and sore throat. At the time of admission, blood pressure was 92/62 mmHg; heart rate, 155/min; respiratory rate, 30/min; and body temperature, 38.6℃. On physical examination, she looked acutely ill and puffy. The conjunctivae were injected. Coarse sounds with inspiratory rales were audible in both lower lung fields. Scattered maculopapular rash was observed on the trunk, and no abdominal mass was palpable. Pretibial pitting edema (1+) was observed.
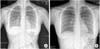
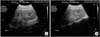
Laboratory measures were as follows: hemoglobin, 13.2 g/dL; hematocrit, 37.8%; white blood cell count, 15,600/µL (neutrophils, 94.1%; lymphocytes, 1.9%); platelet count, 115,000/µL; C-reactive protein, 11.7 mg/dL; blood urea nitrogen (BUN), 44 mg/dL; serum creatinine (Cr), 3.0 mg/dL; sodium, 137 mEq/L; potassium, 4.2 mEq/L; chloride, 100 mEq/L; total CO2, 12 mEq/L; total protein, 5.9 g/dL; albumin, 3.5 g/dL; cholesterol, 91 mg/dL; N-terminal pro-B-type natriuretic peptide (NT-proBNP), 140 pg/mL; prothrombin time, 17.9 s; activated partial thromboplastin time, 48.2 seconds; and D-dimer, 35.2 mg/L. Urinalysis after treatment with initial hydration and furosemide administration showed a specific gravity of 1.005, with protein (2+), and occult blood (3+). The spot urine protein/creatinine ratio was 0.53; creatinine clearance rate, 28.3 mL/min; fractional excretion of sodium, 5.6%; and 24-hour urine protein, 12.2 mg·m-2·h-1. The results of the polymerase chain reaction for nasopharyngeal respiratory viruses were positive for influenza B; the presence of Hantaan virus, Leptospira, and Yersinia antibodies were negative. Bacterial throat culture was negative. Chest radiography revealed hazy increased opacity suggesting pneumonia, and pulmonary edema in both lower lung fields (Fig. 1A), which cleared up on follow-up study (Fig. 1B). Renal ultrasonography showed slightly increased echogenicity in both kidneys, suggestive of renal parenchymal lesions (Fig. 2).
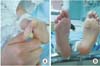
The patient was diagnosed with influenza B virus infection-related pneumonia, dehydration, and AKI. After an intravenous fluid bolus of 0.9% saline (20 mL/kg), intravenous furosemide (2 mg/kg), and mannitol (0.5 g/kg) in sequence, the patient began to produce urine. A continuous intravenous infusion of dopamine (3 µg·kg-1·min-1) was added, and oseltamivir (Tamiflu, 75 mg bid per day for 5 days) was initiated. From the third hospital day (HD), diuresis improved as did renal function (BUN, 41 mg/dL; Cr, 2.4 mg/dL). Her fever resolved, and respiratory symptoms improved. Owing to rapid recovery from AKI, renal biopsy was not performed. Periungual desquamation of both thumbs was first observed on the fourth HD, progressing to peeling of the fingers, toes, and soles (Fig. 3). Serum NT-proBNP was elevated to 1,607 pg/mL. Ophthalmological evaluation of both eyes revealed the presence of anterior uveitis. The echocardiogram showed normal left anterior descending coronary artery measuring 3.6 mm (z-score <2) and the right coronary artery measuring 2.9 mm (z-score <2). Although there was no coronary artery dilation or aneurysm by echocardiography, intravenous immunoglobulin (2 g/kg) was administered for a diagnosis of atypical KD. The clinical course of the patient is shown in Fig. 4. Shortly thereafter, edema disappeared and renal function normalized (Fig. 5).
The patient was discharged in good condition on the 12th HD receiving low-dose aspirin (100 mg per day). At the 2-month follow-up, her renal function remained normal and repeat echocardiography revealed no coronary artery lesions. Shortly afterward, aspirin was discontinued.
Discussion
This is the first case of a simultaneous AKI and atypical KD occurring coincidently with an influenza B virus infection. Although there have been a few reports of influenza A virus H1N1-associated KD [7], some reports of influenza A virus with AKI [123], and one report of influenza B virus-associated rhabdomyolysis with acute renal failure [4], influenza B virus infection with both AKI and KD has not been reported in the literature.
Renal complications of influenza virus infection include AKI, rhabdomyolysis, hemolytic uremic syndrome, and acute glomerulonephritis [2]. The pathophysiology of AKI in influenza virus infection is thought to be multifactorial. Acute tubular necrosis due to hypoperfusion, renal vasoconstriction, and rhabdomyolysis in the setting of a severe systemic inflammatory response with cytokine cascade are likely to occur concurrently [24].
AKI in the 2009 influenza A virus H1N1 pandemic occurred in critically ill patients with respiratory failure who required intensive care [35], and AKI commonly occurs in critically ill patients with other severe infections as well. Therefore, influenza A virus per se may not be an independent risk factor for AKI. During an influenza epidemic in France, 4 of the 9 deaths that occurred were associated with influenza B virus infection [6]. Our patient had influenza B virus-induced pneumonia and AKI. Her respiratory and renal symptoms were not severe and eventually recovered without respiratory or renal support, but the clinical course progressed to atypical KD.
Joshi et al. [7] first reported KD coincident with influenza A infection in 2011. Kim et al. [8] reported the association between KD and respiratory virus infection in 2014, suggesting that influenza A or B virus is the second most common virus associated with KD. However, the pathophysiologic relationship between influenza virus and KD has not been clearly identified.
KD is an acute, self-limited vasculitis of unknown etiology occurring predominantly in infants and young children. The classic diagnosis of KD has been based on the presence of ≥5 days of fever and ≥4 of the following clinical features: (1) bilateral, nonexudative conjunctivitis; (2) polymorphous exanthem; (3) cervical lymphadenopathy; (4) changes in the extremities, including palm and sole erythema, indurative edema of the hands and feet, periungual desquamation; and (5) changes in the oropharynx, including strawberry tongue, red fissured lips, and oropharyngeal erythema [12]. Recently, there has been a trend toward a high incidence of incomplete or atypical KD in which patients do not fulfill the diagnostic criteria. When the patients lack sufficient clinical signs to fulfill the criteria and do not demonstrate atypical clinical features, the term "incomplete" is preferable to "atypical". "Atypical KD" could be reserved for patients who have a problem, such as renal impairment, that generally is not seen in KD [12].
Renal involvement in KD is present in less than 1% of cases [9] and the most common sign of urinary involvement is aseptic pyuria [10]. AKI in KD is uncommon, however, there are a few case reports describing renal disease with atypical KD [1113]. The pathophysiology underlying renal manifestations of KD has been controversial. Some investigators have reported that acute renal failure in KD is due to tubulointerstitial nephropathy with normal glomeruli or mild mesangial expansion, and without vessel involvement [13]. Another investigator described an immune complex-mediated disorder with electron-dense deposits in the mesangial matrix with IgM (3+) and C3 (1+) in the mesangium [11].
Our patient was diagnosed with atypical KD during her clinical course. When renal function had almost recovered, she began to show subacute phase symptom of KD such as periungual desquamation. In scarlet fever, periungual desquamation may also occur. But the presence of uveitis [14] and increased NT-proBNP level [15] strongly favor the diagnosis of KD, even though there was no coronary artery abnormality. In our patient, atypical KD and AKI occurring with influenza B virus infection might be coincidental; we cannot explain the accurate cascade of events. However, AKI due to influenza B virus infection is rare and its concurrent progression to KD has not been previously reported.
In conclusion, we present the case of an unusual association of influenza B virus, AKI, and atypical KD. The precise relationship between these entities remains unknown, but clinicians should be aware of the possibility of their co-occurrence.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download