Abstract
A substantial proportion of adrenal incidentalomas demonstrates subtle hormonal hypersecretion; however, adenomas that cosecrete aldosterone and cortisol are rare. We here report a case of an adrenal mass that was incidentally detected on a computed tomography scan in a 57-year-old man. The patient had a 10-year history of diabetes mellitus and a 5-year history of hypertension. Evaluation revealed hyperaldosteronemia with an elevated plasma aldosterone-to-renin ratio, hypokalemia, unsuppressed cortisol after dexamethasone administration, and elevated urinary free cortisol concentration. The appearance of the right adrenalectomy specimen indicated adrenal adenoma. Postoperatively, the blood glucose and blood pressure control improved and the urinary cortisol and aldosterone-to-renin ratio normalized. A complete endocrine evaluation in patients with incidentally discovered adrenal masses should be performed, even if the patient has a long-standing history of hypertension and diabetes, to avoid any postoperative adrenal crises.
As the frequency of performing abdominal computed tomography (CT) scanning is increasing, owing to the increased quality of medical services, the prevalence of adrenal incidentalomas is also rising. Although most adrenal incidentalomas are benign and nonfunctioning, a substantial proportion demonstrates subtle hormonal hypersecretion. Adrenal adenomas mainly manifest as autonomous cortisol secretion, with a reported prevalence ranging from 1% to 47%, depending on the applied diagnostic criteria [1]. However, adrenal tumors secreting aldosterone and cortisol simultaneously are rare, especially among incidentalomas [23].
The recognition of cortisol and aldosterone cosecreting adrenal adenomas will have implications for the perioperative management, and is necessary for administering glucocorticoid treatment to avert the possibility of adrenal crisis [4]. Herein, we present a case of an incidentally detected adrenal adenoma secreting excess cortisol and aldosterone and discuss how this case was managed.
A 57-year-old man presented with a right adrenal mass that was detected on an abdominal CT scan in a routine health examination. The patient had 10-year history of type 2 diabetes mellitus and a 5-year history of hypertension. He took glimepiride (4 mg), metformin (1 g), vildagliptin (50 mg) and amlodipine (5 mg) twice daily to manage his diabetes and blood pressure. His father had hypertension and diabetes, and his mother and 3 of 4 siblings also had diabetes. On admission, his blood pressure was 143/94 mmHg; pulse, 75 beats/min; respiratory rate, 20 breaths/min; weight, 72.3 kg; height, 164 cm; and body mass index, 26.88 kg/m2. He did not show features of Cushing syndrome, including easy bruising, fat deposition on the abdomen and neck, or purple striae.
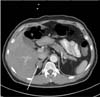
Abdominal CT scan showed a well-circumscribed and homogeneous 2.5×2.0 cm right adrenal mass, with a density of 60.4 Hounsfield units (HU) (Fig. 1). The biochemical analysis showed the following results: sodium, 142 mEq/L; potassium, 3.1 mEq/L; chloride, 98 mEq/L; total carbon dioxide 29 mEq/L; calcium, 8.8 mg/dL; phosphorus, 3.2 mg/dL; blood urea nitrogen, 10 mg/dL; serum creatinine, 0.9 mg/dL; fasting plasma glucose, 90 mg/dL; and glycated hemoglobin (HbA1C), 7.3% (Table 1).
Two weeks after switching the patient's treatment from amlodipine to doxazosin, the patient underwent endocrine evaluation for the differential diagnosis of adrenal incidentaloma. The plasma aldosterone concentration (PAC) was 21.7 ng/dL, with a plasma renin activity (PRA) of 0.04 ng/mL/hr and an aldosterone-to-renin ratio (ARR) of 542.5 ng/dL:ng/mL/hr. The early-morning plasma adrenocorticotrophic hormone (ACTH) concentration was <1.0 pg/mL (normal, 7.2 to 63.3 pg/mL), with a serum cortisol concentration of 9.3 µg/dL (normal, 6.5 to 19.5 µg/dL) and urinary free cortisol concentration of 452.4 µg/day. The patient underwent a 1 mg overnight dexamethasone suppression test, during which the serum cortisol concentration decreased to 8.8 µg/dL, which is greater than the 1.8 µg/dL cutoff to rule out Cushing syndrome based on the current Endocrine Society guidelines [5]. According to the Endocrine Society guidelines [6], pheochromocytoma was also ruled out on the basis of normal 24 hr urinary catecholamine and metanephrine concentrations.
2. Treatment and follow-up In most circumstances, isolated primary aldosteronism is confirmed with a salt suppression test, followed by adrenal venous sampling for the purpose of lateralization in preparation for surgery [78]. However, because we confirmed cosecretion of cortisol and aldosterone, a laparoscopic right adrenalectomy was carried out without further examination.
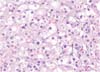
A section of the specimen obtained showed a well-circumscribed, golden-yellow mass in the adrenal cortex, measuring 1.8×1.2 cm. On low-power examination, the mass was surrounded by a thin fibrous capsule without capsular and vascular invasion. The tumor cells were arranged in a nest and trabeculae pattern, and no necrosis was identified. On high-power examination, the tumor cells showed abundant and eosinophilic cytoplasm admixed with clear, vacuolated cytoplasm. The nuclei were uniform with rare mitoses and pleomorphism. Spironolactone bodies were noted; these are concentric laminated eosinophilic inclusions known to be found in the zona glomerulosa cells secondary to spironolactone treatment [9]. According to the Weiss scoring system [10], the final diagnosis was benign adrenocortical adenoma (Fig. 2).
On the day of surgery, 200 mg of intravenous hydrocortisone was injected to avoid adrenal crisis, and then gradually tapered to oral prednisolone at 5 mg daily. One week after the operation, repeat testing showed a PAC of 12.55 ng/dL and PRA of 0.19 ng/mL/h4, with an ARR of 66.1 ng/dL:ng/mL/hr, ACTH of 6.9 pg/mL, and cortisol concentration of 1.9 µg/dL. Twelve months after the operation, the cortisol concentration was increased to 9.1 µg/dL; ACTH, 28.8 pg/mL; PAC, 14.8 ng/dL; PRA, 0.6 ng/mL/hr; and ARR, 24.6 ng/dL:ng/mL/hr, indicating resolution of the primary aldosteronism and cortisol deficiency (Table 1). Thus, steroid replacement was discontinued and the patient no longer required spironolactone or potassium supplements. Long-term follow-up of the patient showed improved blood pressure and glucose control. Therefore, the doses of amlodipine, metformin, and glimepiride were decreased (Table 1).
We here described a case of cosecretion of aldosterone and cortisol by a benign adrenal adenoma that was incidentally discovered on an abdominal CT scan. Such a case has not been previously reported in Korea, as all reported cosecreting adenomas were diagnosed as a result of evaluation for presenting symptoms when the patients were hospitalized. The present patient had a history of diabetes and hypertension, which were exacerbated by the excess aldosterone and cortisol. Nevertheless, our index of suspicion for aldosterone and cortisol production was not high, owing to his family history of diabetes and hypertension and the high prevalence of these diseases among the general population. The excessive secretion of aldosterone and subclinical hypercortisolism were confirmed after additional adrenal adenoma workup.
Aldosterone and cortisol cosecretion should be assessed in the initial workup for adrenal adenomas, even though this is a rare condition, because the diagnosis can influence the treatment options, especially in terms of the postsurgical management. After the removal of a tumor that secretes glucocorticoids, patients may develop adrenal insufficiency secondary to the suppression of the hypothalamic-pituitary-adrenal axis [11]. As in the case presented here, hypocortisolism can develop postoperatively and glucocorticoid replacement should be considered in accordance with the degree of adrenal insufficiency.
The vast majority of cases of aldosterone and cortisol cosecreting adenomas have been described in females, with an average diameter of 26 mm and presenting with hypertension combined with hypokalemia [12]. While the accurate prevalence of aldosterone and cortisol cosecreting adenomas is not known, it has been estimated that 14% of patients with primary aldosteronism have cosecreting tumors [13]. Aldosterone-producing adenomas that solely consist of zona glomerulosa cells are very rare, and these tumors are usually composed of different cell types. This implies that such tumors have the potential to cosecrete both aldosterone and cortisol. A greater proportion of cortisol-producing cells compared to pure aldosterone-producing adenoma cells or a large tumor size, which would enable the production of detectable amounts of cortisol, are considered to contribute to the development of cosecreting adrenal adenomas.
In the present case, we noted improved blood glucose and blood pressure control after the operation; accordingly, the doses of amlodipine and oral hypoglycemic agents needed to achieve the optimal blood glucose and blood pressure targets could be decreased (Table 1). The presence of hypertension, diabetes mellitus, osteopenia, and fatty liver implies the effects of long-term exposure to cortisol and aldosterone although the patient did not show external features of Cushing's syndrome. Fortunately, he did not present cardiovascular or cerebrovascular diseases which are important adverse outcomes of cortisol and aldosterone excess.
Hypokalemia was the only indicator of aldosterone excess. In fact, the patient showed hypokalemia (K 3.0 mEq/L) 3 years before the detection of the adrenal incidentaloma in a routine health check. Thus, if hormonal screening had been performed at that time, the adrenal adenoma would have potentially been diagnosed earlier.
A limitation of our study is the absence of adrenal venous sampling data and molecular biological confirmation of aldosterone and cortisol cosecretion. However, a unilateral mass was clearly visible on the CT scan, and laboratory data indicated both aldosterone and cortisol excess. Taken together, these findings provided a solid basis to confirm the diagnosis of a functioning unilateral adrenal adenoma.
We here presented a case of a benign adrenal adenoma cosecreting excess aldosterone and cortisol in a patient with a longstanding history of diabetes and hypertension. It is important to recognize cosecreting adrenal adenomas to avoid adrenal crisis or symptomatic adrenal insufficiency by optimal glucocorticoid replacement after surgery. Thus, complete evaluation should be performed in patients with incidentally discovered adrenal masses.
Figures and Tables
Fig. 1
Abdominal computed tomography findings. A 2.5×2.0-cm well-circumscribed and homogeneous mass with a density of 60.4 Hounsfield units is seen at the right adrenal gland (arrow).
References
1. Tsagarakis S, Vassiliadi D, Thalassinos N. Endogenous subclinical hypercortisolism: Diagnostic uncertainties and clinical implications. J Endocrinol Invest. 2006; 29:471–482.
2. Oh JY. How to approach and follow adrenal incidentaloma? Korean J Intern Med. 2013; 28:541–543.
3. Kim BY, Chun AR, Kim KJ, Jung CH, Kang SK, Mok JO, et al. Clinical Characteristics and Metabolic Features of Patients with Adrenal Incidentalomas with or without Subclinical Cushing's Syndrome. Endocrinol Metab (Seoul). 2014; 29:457–463.
4. Yoon V, Heyliger A, Maekawa T, Sasano H, Carrick K, Woodruff S, et al. Benign adrenal adenomas secreting excess mineralocorticoids and glucocorticoids. Endocrinol Diabetes Metab Case Rep. 2013; 2013:130042.
5. Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, et al. The diagnosis of Cushing's syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008; 93:1526–1540.
6. Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SK, Murad MH, et al. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014; 99:1915–1942.
7. Young WF, Stanson AW, Thompson GB, Grant CS, Farley DR, van Heerden JA. Role for adrenal venous sampling in primary aldosteronism. Surgery. 2004; 136:1227–1235.
8. Funder JW, Carey RM, Fardella C, Gomez-Sanchez CE, Mantero F, Stowasser M, et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008; 93:3266–3281.
9. Patel KA, Calomeni EP, Nadasdy T, Zynger DL. Adrenal gland inclusions in patients treated with aldosterone antagonists (Spironolactone/Eplerenone): incidence, morphology, and ultrastructural findings. Diagn Pathol. 2014; 9:147.
10. Aubert S, Wacrenier A, Leroy X, Devos P, Carnaille B, Proye C, et al. Weiss system revisited: a clinicopathologic and immunohistochemical study of 49 adrenocortical tumors. Am J Surg Pathol. 2002; 26:1612–1619.
11. Chang KY, Ryu S, Cho JY, Kim HW. Aldosterone- and cortisol-coproducing adrenal adenoma without clinical features of Cushing syndrome. Korean J Intern Med. 2014; 29:679–682.
12. Spath M, Korovkin S, Antke C, Anlauf M, Willenberg HS. Aldosterone-and cortisol-co-secreting adrenal tumors: the lost subtype of primary aldosteronism. Eur J Endocrinol. 2011; 164:447–455.
13. Lau JH, Sze WC, Reznek RH, Matson M, Sahdev A, Carpenter R, et al. A prospective evaluation of postural stimulation testing, computed tomography and adrenal vein sampling in the differential diagnosis of primary aldosteronism. Clin Endocrinol (Oxf). 2012; 76:182–188.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download