Abstract
A 56-year-old man presented with sudden onset of congestive heart failure (New York Heart Association class III to IV) after mild stress and developed various cardiovascular manifestations. At first visit, cardiac enzyme elevation, regional left ventricular (LV) wall motion abnormality and pulmonary edema were evident. However, coronary angiography was normal. LV function was totally recovered at discharge, suspicious of fulminant myocarditis. During the hospital stay, acute non-obstructive stroke without neurologic sequelae occurred. After 3 years, he re-admitted because ventricular tachycardia and severe LV systolic dysfunction (ejection fraction, 15%) were developed. After 3 days of applying percutaneous cardiopulmonary bypass system, the patient was completely recovered. Suspicious of pheochromocytoma, we checked 24-hour urine catecholamines and metanephrines and abdomen computed tomography, which revealed pheochromocytoma. The patient underwent laparoscopic adrenalectomy.
Excess concentrations of catecholamines in pheochromocytoma can cause various clinical manifestations. There are few reports of pheochromocytoma presented with stress-induced cardiomyopathy (catecholamine-induced cardiomyopathy) after mild stress and the case of pheochromocytoma associated with non-occlusive stroke is more uncommon.
We report a case of pheochromocytoma manifested repeated stress-induced cardiomyopathy after mild stress featuring fulminant myocarditis and combined with non-occlusive stroke and ventricular tachycardia (VT).
A 56-year-old man who had no underlying disease, but who had experienced mild stress for a month, visited the emergency room (ER) of another hospital because of headache and abdominal pain 1 day before admission. Systolic and diastolic blood pressures (BPs) were 180/100 mmHg. The patient received prescriptions for hypertension and dyspepsia, and was sent home. Six hours later, headache and vomiting developed, prompting revisit to the ER. Pulmonary edema and elevations in creatine phosphokinase (CK)-MB and troponin-T were detected. The patient was admitted to the intensive care unit overnight and was transferred to our hospital the next day.
The patient was a social drinker and non-smoker. Cardiomegaly and pulmonary edema were evident on chest radiography and an abdominal X-ray revealed paralytic ileus. ECG showed non-specific ST change at inferior and lateral leads, and borderline QT prolongation. Laboratory findings revealed leukocytosis, and an elevated cardiac enzyme profile consisting of WBC 21.10×106/L (normal range, 4.0~10.0×106/L), CK 363 U/L (normal range, 26~140 U/L), CK-MB 26.2 ng/mL (normal, <3.61 ng/mL), and N-terminal prohormone of brain natriuretic peptide (NT pro-BNP) 6,388 pg/mL (normal range, 0~125 pg/mL). Transthoracic echocardiography (TTE) showed mild left ventricular (LV) dysfunction (ejection fraction [EF], 45~50%) (Fig. 1), regional wall motion abnormality (RWMA) suggestive of multi-vessel coronary artery disease and LV hypertrophy, and left atrial enlargement. Coronary angiography (CAG) showed no significant stenosis at three major coronary vessels.
He was treated conservatively. Two days later, cardiac MRI demonstrated near-normal LV function and no myocardial damage. On the same day, the patient exhibited dysarthria. A brain CT, angiography, and MRI showed no major vessel obstruction but acute infarction on the body of the right corpus callosum (Fig. 2). By day 11 of hospitalization, dysarthria was fully recovered. The patient was discharged the next day. Eight months later, TTE showed normal LV function and totally recovered RWMA (Fig. 1). The patient took aspirin, clopidogrel, perindopril, furosemide, aldactone, trimetazidine, and bisoprolol for one year, followed by aspirin, clopidogrel, perindopril through an outpatient clinic.
Three years after the first admission, the patient was admitted via the ER for epigastric discomfort, nausea, and headache accompanied by dyspnea and cold sweating. On physical examination, systolic and diastolic BPs were 200/100 mmHg, heart rate was 170 beats per minute, respiratory rate was 24. Chest radiography showed cardiomegaly and pulmonary edema. Initial ECG showed VT; after amiodarone loading, the rhythm returned to sinus rhythm and ST segment elevation on lead V3~6 was evident (Fig. 3). Laboratory findings were as follows: leukocytosis (WBC 12.4×106/L); slightly elevated NT pro-BNP (221.3 pg/mL); elevated D-dimer 3.53 mg/L (normal range, 0~0.55 mg/L); respiratory acidosis; arterial blood gas values of pH 7.241, PaCO2 37.7 mmHg, PaO2 42.9 mmHg, bicarbonate 16.3, and oxygen saturation 90.3%. Levels of CK, CK-MB, and troponin-T were within normal ranges. TTE showed severe LV systolic dysfunction, EF of 10%~15%, and RWMA with severe hypokinesia and akinesia (Fig. 2). With the support of external bypass system (EBS) and intraaortic balloon pump, an emergency CAG was performed, there were no obstructive vessels. EBS was continued for 3 days, thereafter LV contractility and wall motion was improved (EF, 50~55%) other than apical akinesia. Hospital day 12, cardiac function was fully recovered (LVEF, 72%) without RWMA.
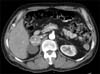
We performed laboratory work-up for pheochromocytoma before discharge. Twenty-four hour urine metanephrine, normetanephrine, vanillylmandelic acid (VMA), and catecholamine were elevated: metanephrine 3,299.7 µg/day (normal range, 52.0~341.0 µg/day), normetanephrine 2,933.3 µg/day (normal range, 88.0~444.0 µg/day), VMA 13.5 mg/day (normal range, 0~8 mg/day), epinephrine 172.4 µg/day (normal range, 0~20 µg/day), and norepinephrine 193.7 µg/day (normal range, 15~80 µg/day) (Table 1). Abdomen CT demonstrated a 4-cm sized adrenal mass on the right adrenal gland (Fig. 4), and positron emission tomography (PET) revealed a tumorous condition in the right adrenal gland and no abnormal fludeoxyglucose uptake at other sites. A work-up for multiple endocrine neoplasia type 2 demonstrated no specific findings on laboratory study and thyroid sonography, except for subclinical hypothyroidism evident as thyroid-stimulating hormone 9.350 µIU/mL, free T4 1.3 ng/dL, and T3 1.1 ng/mL.
We concluded that the definite diagnosis was catecholamine-induced cardiomyopathy due to pheochromocytoma. The patient underwent laparoscopic right adrenalectomy after 2 weeks use of alpha-blocking agent. Previous elevated levels of 24-hour urine metanephrine and normetanephrine level were normalized after surgery (Table 1).
Pheochromocytoma typically presents with the classic signs and symptoms of paroxysmal hypertension, tachycardia, and episodic headache in young adults. Cardiomyopathy and heart failure are relatively uncommon. There are several reports of pheochromocytoma presenting with takotsubo or stress-induced cardiomyopathy. However, manifestation as recurrent catecholamine-induced cardiomyopathy, combined with non-occlusive stroke and VT, as in this case, is rare.
Stress-induced cardiomyopathy resembles an acute coronary syndrome, but completely reversible myocardial dysfunction occurs in the absence of coronary thrombosis. Plasma catecholamine levels can be markedly elevated in some patients with stress cardiomyopathy and intravenous catecholamines can precipitate stress cardiomyopathy [1]. The precise mechanism by which excessive sympathetic stimulation may result in transient LV dysfunction is controversial. Abnormal myocardial blood flow due to sympathetically mediated microvascular dysfunction has been suggested. An alternative explanation is the direct effect of catecholamines on cardiac myocytes, possibly through cyclic adenosine monophosphate-mediated calcium overload [2].
In our case, the patient experienced dysarthria and acute cerebral infarction, but no significant stenosis of intracranial vessels. Only a few reports mention strokes occurring in the background of pheochromocytoma. Most of cases are ischemic strokes rather than hemorrhagic strokes. Dagartzikas et al. [3] described an intracardiac mural thrombus that developed because of cardiomyopathy secondary to excessive catecholamine excretion, which resulted in cardiogenic cerebral embolism. Hill and Schwartzman [4] and Oh et al. [5] stated that the cause of cerebral infarction is vasospasm due to excessive catecholamines. Van et al. [6] stated that the cause of stroke is cerebral thrombosis because of persistent hypertension. Given these results, we favor vasospasm as the underlying etiology of the patient's neurologic deficits.
Several cases of VT associated with pheochromocytoma had been reported recently [7,8,9,10]. VT is a rare complication of pheochromocytoma. VT was reported in two patients among 145 pheochromocytoma subjects [11]. VT was presently terminated by administration of intravenous amiodarone. However, another report described VT recurrence even with the use of amiodarone, and the resolution of recurrence after adrenalectomy [7].
In conclusion, we report a 56-year-old male diagnosed with recurrent catecholamine-induced cardiomyopathy due to pheochromocytoma, accompanying stroke and VT. When idiopathic transient LV dysfunction was detected, pheochromocytoma should be suspected. This is a rare case of pheochromocytoma presenting with diverse uncommon cardiovascular complication.
Figures and Tables
Fig. 1
Electrocardiogram (ECG), chest X-ray and transthoracic echocardiography (TTE) findings at first admission. Initially, (A) ECG shows non-specific ST change, and (B) chest X-ray shows pulmonary edema. (C-1) TTE reveals mild left ventricular (LV) systolic dysfunction, but (C-2) eight month follow-up TTE after first admission demonstrates complete recovery of LV systolic function.
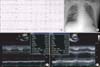
Fig. 2
Brain imaging studies at first admission. Brain computer tomography (CT) (A), brain CT angiography (B), brain magnetic resonance imaging (MRI), diffusion weighted image (C), brain MRI, fluid attenuation inversion recovery (FLAIR) image (D), and brain MRI, apparent diffusion coefficient (ADC) map (E) shows acute infarction on right corpus callosum without major vessel obstruction.
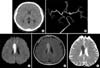
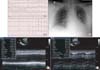
Fig. 3
Electrocardiogram (ECG), chest X-ray and transthoracic echocardiography (TTE) findings at second admission. Initial ECG (A-1) shows ventricular tachycardia (VT). VT is resolved (A-2) by loading of amiodarone, and ST segment elevation appears on V3?V6 leads. (B) Chest X-ray (anteroposterior image) shows pulmonary edema. (C-1) Initial echocardiography shows severe left ventricular (LV) dysfunction. (C-2) Follow-up echocardiography reveals completely normalized LV systolic function on hospital day 12.
References
1. Abraham J, Mudd JO, Kapur NK, Klein K, Champion HC, Wittstein IS. Stress cardiomyopathy after intravenous administration of catecholamines and beta-receptor agonists. J Am Coll Cardiol. 2009; 53:1320–1325.
2. Wittstein IS. Stress cardiomyopathy: a syndrome of Catecholamine-mediated myocardial stunning? Cell Mol Neurobiol. 2012; 32:847–857.
3. Dagartzikas MI, Sprague K, Carter G, Tobias JD. Cerebrovascular event, dilated cardiomyopathy, and pheochromocytoma. Pediatr Emerg Care. 2002; 18:33–35.
4. Hill JB, Schwartzman RJ. Cerebral infarction and disseminated intravascular coagulation with pheochromocytoma. Arch Neurol. 1981; 38:395.
5. Oh HC, Koh JM, Kim MS, Park JY, Shong YK, Lee KU, et al. A case of ACTH-producing pheochromocytoma associated with pregnancy. Endocr J. 2003; 50:739–744.
6. Van YH, Wang HS, Lai CH, Lin JN, Lo FS. Pheochromocytoma presenting as stroke in two Taiwanese children. J Pediatr Endocrinol Metab. 2002; 15:1563–1567.
7. Park JW, Park SJ, Hur KY, Kim JH, Choi YL, Park SM, et al. Recurrent ventricular tachycardia in malignant metastatic pheochromocytoma. Circulation. 2012; 125:e435–e438.
8. Huang YC, Chang CH, Wang CH, Chang JS. Pheochromocytoma complicated with severe ventricular tachycardia: report of one case. Acta Paediatr Taiwan. 2007; 48:280–284.
9. Traykov VB, Kotirkov KI, Petrov IS. Pheochromocytoma presenting with bidirectional ventricular tachycardia. Heart. 2013; 99:509.
10. Gregori M, Paneni F, D'Agostino M, Tocci G, Ferrucci A, Savoia C. High blood pressure, ventricular tachycardia and transient left ventricular dysfunction: do not forget pheochromocytoma. High Blood Press Cardiovasc Prev. 2011; 18:57–59.
11. Zelinka T, Petrak O, Turkova H, Holaj R, Strauch B, Krsek M, et al. High incidence of cardiovascular complications in pheochromocytoma. Horm Metab Res. 2012; 44:379–384.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download