Abstract
Among the possible venous thromboembolic events in nephrotic syndrome, renal vein thrombosis and pulmonary embolism are common, while portal vein thrombosis (PVT) is rare. This report describes a 26-year-old man with histologically proven minimal change disease (MCD) complicated by PVT. The patient presented with epigastric pain and edema. He had been diagnosed with MCD five months earlier and achieved complete remission with corticosteroids, which were discontinued one month before the visit. Full-blown relapsing nephrotic syndrome was evident on laboratory and clinical findings, and an abdominal computed tomography revealed PVT. He immediately received immunosuppressants and anticoagulation therapy. An eight-week treatment resulted in complete remission, and a follow-up abdominal ultrasonography showed disappearance of PVT. In conclusion, PVT is rare and may not be easily diagnosed in patients with nephrotic syndrome suffering from abdominal pain. Early recognition of this rare complication and prompt immunosuppression and anticoagulation therapy are encouraged to avoid a fatal outcome.
Thromboembolic events are considered to be critical complications of nephrotic syndrome. Much evidence suggests that nephrotic syndrome is associated with a hypercoagulable state. Altered coagulation factor profiles and increased viscosity due to volume depletion may play a role in thrombus formation. The renal vein and deep vein of the lower limbs are most frequently involved, which often lead to acute kidney injury and pulmonary embolism, respectively. The portal vein, however, is a rare site of thrombus formation in patients with nephrotic syndrome and may be misdiagnosed when physicians assess abdominal pain in these patients. This report describes a case of histologically proven minimal change disease (MCD) complicated by portal vein thrombosis.
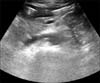
A 26-year-old male visited our hospital due to sudden nausea, vomiting, and epigastric pain. Five months before the visit, he was diagnosed with MCD by renal biopsy. Complete remission was achieved after treatment with oral corticosteroids at an initial dose of 1 mg/kg/day, followed by a slow taper over the next four months. Blood pressure was 101/76 mmHg, heart rate 72/min, respiratory rate 16/min, and body temperature 37.4℃. On physical examination, the abdomen was tense and rigid. There was direct tenderness in the epigastric abdominal area without rebounding tenderness. Pretibial pitting edema and ascites were also noted, but his mouth and tongue were dry. Initial laboratory tests showed the following values: hemoglobin, 20.2 g/dL; hematocrit, 58.6%; WBC, 18,890/µL; platelet, 152,000/µL; prothrombin time, 0.96 international normalized ratio (INR); activated partial thromboplastin time, 29.9 seconds; serum creatinine, 1.39 mg/dL; estimated glomerular filtration rate (eGFR), 61.8 mL/min/1.73 m2 [eGFR modification of diet in renal disease study (MDRD) (mL/min/1.73 m2)=175×(serum creatinine)-1.154×(age)-0.203×(0.742 if female)]; blood urea nitrogen, 13.9 mg/dL; sodium, 139 mEq/L; potassium, 3.3 mEq/L; chloride, 104 mEq/L; total protein, 4.4 g/dL; albumin, 1.7 g/dL; cholesterol, 472 mg/dL; alkaline phosphate, 58 IU/L; aspartate aminotransferase, 25 IU/L; alanine aminotransferase, 21 IU/L; amylase, 39 U/L; and lipase, 18 U/L. The spot urine protein/urine creatinine ratio (UPCR) was 17.20 and 24-hr proteinuria was 25.3 g/day. Random urine sodium was 60 mEq/L and fractional excretion of sodium (FENa) was 0.99%. Chest X-ray films showed bilateral pleural effusion and mild pulmonary edema. Abdominal computed tomography (CT) and ultrasonography performed on admission showed thrombosis involving the main and right portal vein without cavernous transformation, suggesting an acute phase of thrombosis (Fig. 1). The patient immediately received anticoagulation therapy with intravenous heparin infusion, followed by oral warfarin. We adjusted the warfarin dose to maintain a target INR between 2.0 and 3.0. In addition, high-dose oral prednisolone at a dose of 1 mg/kg/day was administered to treat relapse of nephrotic syndrome. A two-week corticosteroid treatment failed to significantly reduce proteinuria (19 g/day) and the patient was not tolerant to the side effects of steroid, thus cyclosporine at 5 mg/kg/day was added. One month later, 24-hr proteinuria decreased to 0.52 g/day and kidney function recovered (eGFR>90 mL/min/1.73 m2). Complete remission was achieved after six weeks of cyclosporine use. Follow-up abdominal ultrasonography eight weeks after the onset of portal vein thrombosis showed a patent portal vein without thrombus (Fig. 2). Anticoagulation therapy was maintained for one additional month and was then discontinued. Cyclosporine at a reduced dose of 2.0 mg/kg/day was maintained during the following 24 months and was tapered to 1.0 mg/kg/day. The patient has remained in remission without thromboembolic events.
Nephrotic syndrome increases the risk of developing venous thrombosis in approximately 25% of patients [1]. The mechanisms underlying such a hypercoagulable state are deficiency of endogenous antithrombotic factors, including antithrombin III and proteins C and S due to urinary loss; increased formation of factors that promote thrombosis including factor V and VIII, von Willebrand factor, fibrinogen, and plasminogen activator inhibitor 1; and impaired thrombolytic activity, such as decreased plasminogen levels [1,2,3,4]. In addition, hypovolemia-induced hyperviscosity, hypercholesterolemia, hypoalbuminemia, and the use of corticosteroid are also associated with the development of thromboembolic events [5,6,7].
Thromboembolic events most commonly involve the renal vein, deep vein of the lower limbs in patients with the greatest risk seen in membranous glomerulopathy, menbranoproliferative glomerulonephritis, and minimal change disease among subtypes of nephrotic syndrome [2,3]. Portal vein thrombosis is rare. A review of the literature identified nine cases of portal vein thrombosis to date (Table 1) [8,9,10,11,12,13,14,15,16]. All occurred in the setting of severe hypoalbuminemia (1.5 to 2.1 g/dL), heavy proteinuria (3.46 to 25 g/day), high ratio of proteinuria to serum albumin, hypercholesterolemia (360 to 549 mg/dL), and polycythemia (48% to 56%) secondary to hypovolemia when nephrotic syndrome was first diagnosed or relapsed. Most cases of portal vein thrombosis occurred in MCD. Seven cases (77.8%) were histologically proven by renal biopsy, while the others were clinically presumed to have MCD due to a rapid response to steroid therapy, but renal biopsy was not performed. In line with these findings, our patient clearly had a relapse of MCD and exhibited a markedly hypovolemic status presenting polycythemia (58.6%) before the use of diuretics and full-blown nephrotic features such as heavy proteinuria (25.3 g/day), hypoalbuminemia (1.7 g/dL), high ratio of proteinuria to serum albumin, and hypercholesterolemia (472 mg/dL). However, why portal vein thrombosis is more common in MCD remains unclear because these features are common in all subtypes of nephrotic syndrome. The more severe clinical nephrotic features combined with hyperviscosity secondary to hypovolemia might contribute to the increased tendency for thromboembolism irrespective of nephrotic syndrome subtype. Further studies are required to delineate whether MCD is more predisposing to portal vein thrombosis than other subtypes.
Portal venous thrombosis is a rare condition, thus it cannot be easily diagnosed when patients with nephrotic syndrome present with abdominal pain. Conventionally, gastrointestinal manifestations such as abdominal pain, nausea, and vomiting are considered as 'nephrotic crisis.' Although the mechanisms responsible for this are not clear, severe volume depletion associated with full-blown nephrotic status can cause bowel ischemia. This condition can be treated by adequate intravenous fluid resuscitation. However, in cases of portal vein thrombosis, this therapy alone is not sufficient, and persistently unresolved portal hypertension may lead to a fatal outcome. Therefore, portal vein thrombosis should be considered in the differential diagnosis when assessing abdominal pain in patients with nephrotic syndrome. In addition, imaging studies such as an abdominal ultrasonography or CT scan should be encouraged to detect this rare complication.
Spontaneous recanalization after portal vein thrombosis is unlikely, but recanalization occurs in about 40% of patients treated with anticoagulation therapy, which can prevent further thrombosis and development of portal hypertension and its complications [17,18]. Timing of the initiation of anticoagulation therapy is important because the probability of recanalization decreases from 69% when anticoagulation is initiated within the first week after diagnosis to 25% when initiated in the second week [18,19]. Although there is currently no pre-set guideline on anticoagulation therapy in patients with nephrotic syndrome complicated by thromboembolic events, immediate anticoagulation treatment with unfractionated or low molecular heparin and oral warfarin is recommended in symptomatic portal vein thrombosis. Also, anticoagulant therapy should be maintained for the duration of nephrotic syndrome as long as the thromboembolic risk persists [3]. Furthermore, achieving remission of nephrotic syndrome with immunosuppression is of paramount importance to recover the altered coagulation profiles.
In conclusion, portal vein thrombosis is rare and may not be easily diagnosed in patients with nephrotic syndrome suffering from abdominal pain. Early recognition of this rare complication and prompt anticoagulation therapy and immunosuppression are emphasized to avoid a fatal outcome.
Figures and Tables
 | Fig. 1Imaging studies for portal vein thrombosis. (A) Transverse and (B) coronal views of an abdominal computed tomography scan clearly show portal vein thrombosis (arrow). (C) Thrombi are also noted in an abdominal ultrasonography (arrow). |
References
1. Kerlin BA, Ayoob R, Smoyer WE. Epidemiology and pathophysiology of nephrotic syndrome-associated thromboembolic disease. Clin J Am Soc Nephrol. 2012; 7:513–520.
2. Singhal R, Brimble KS. Thromboembolic complications in the nephrotic syndrome: pathophysiology and clinical management. Thromb Res. 2006; 118:397–407.
3. Glassock RJ. Prophylactic anticoagulation in nephrotic syndrome: a clinical conundrum. J Am Soc Nephrol. 2007; 18:2221–2225.
4. Loscalzo J. Venous thrombosis in the nephrotic syndrome. N Engl J Med. 2013; 368:956–958.
5. Ozsoylu S, Strauss HS, Diamond LK. Effects of corticosteroids on coagulation of the blood. Nature. 1962; 195:1214–1215.
6. Bostom AG, Shemin D. Abnormalities of lipoprotein metabolism in the nephrotic syndrome. N Engl J Med. 1991; 324:697–698.
7. Fahal IH, McClelland P, Hay CR, Bell GM. Arterial thrombosis in the nephrotic syndrome. Postgrad Med J. 1994; 70:905–909.
8. Woolf AS, Street PR, Walmsley KM, Cohen SL. Portal vein thrombosis in the nephrotic syndrome. Nephrol Dial Transplant. 1989; 4:581–582.
9. De Luca M, Dugo M, Arduini R, Liessi G. Acute venous thrombosis of spleno-mesenteric portal axis: an unusual localization of thromboembolism in the nephrotic syndrome. Am J Nephrol. 1991; 11:260–263.
10. Plaisier EM, Legallicier B, Faintuch JM, Ronco PM. Acute portal vein thrombosis at the onset of a nephrotic syndrome. Nephrol Dial Transplant. 1996; 11:696–698.
11. Etoh Y, Ohsawa I, Fujita T, Fuke Y, Endo M, Ohi H, et al. Nephrotic syndrome with portal, splenic and renal vein thrombosis. A case report. Nephron. 2002; 92:680–684.
12. Varghese J, Mathew A, Seethalekshmy NV, Kurian G, Unni VN. Isolated portal vein thrombosis in nephrotic syndrome. Indian J Nephrol. 2007; 17:26–28.
13. Sun L, Xu C. Portal vein thrombosis as the first sign of nephrotic syndrome. Nat Clin Pract Nephrol. 2008; 4:342–345.
14. Bian F, Ge QM, Jiang GR, Wen LP. Cavernous transformation of the portal vein in nephrotic syndrome. Vasa. 2011; 40:323–326.
15. Wang YC, Chuang FR, Lee WC, Chen TC, Ko SF, Wang IK, et al. Low-molecular-weight heparin successfully used to treat a nephrotic patient complicated by superior mesenteric vein thrombosis and portal vein thrombosis. Med Princ Pract. 2011; 20:196–199.
16. Wang J, Fan Q, Chen Y, Dong X, Zhang Y, Feng J, et al. A case report of minimal change nephrotic syndrome complicated with portal, splenic and superior mesenteric vein thrombosis. Clin Nephrol. 2012; 77:505–509.
17. Sogaard KK, Astrup LB, Vilstrup H, Gronbaek H. Portal vein thrombosis; risk factors, clinical presentation and treatment. BMC Gastroenterol. 2007; 7:34.
18. Turnes J, Garcia-Pagan JC, Gonzalez M, Aracil C, Calleja JL, Ripoll C, et al. Portal hypertension-related complications after acute portal vein thrombosis: impact of early anticoagulation. Clin Gastroenterol Hepatol. 2008; 6:1412–1417.
19. Condat B, Pessione F, Helene Denninger M, Hillaire S, Valla D. Recent portal or mesenteric venous thrombosis: increased recognition and frequent recanalization on anticoagulant therapy. Hepatology. 2000; 32:466–470.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download