Abstract
Sunitinib an inhibitor of the vascular endothelial growth factor receptor, is highly effective against renal cell carcinoma and is now widely used in patients with metastatic disease. Gastroesophageal reflux disease (GERD) is rarely reported as a side effect of sunitinib. We report two cases of GERD with upper gastrointestinal bleeding related to sunitinib administration. Both cases responded well to conservative management. Microscopic findings in both cases showed cellular atypia such as hyperchromasia, increases in nuclear size, and multinucleation. The cellular atypia of the squamous mucosa appears to be associated with reparative processes.
Angiogenic inhibitors such as sunitinib have been found to increase survival and are approved for treatment of advanced renal cell carcinoma (RCC). However, drugs of this class may cause cutaneous, vascular, and mucosal toxicities, including hand-foot skin reaction, skin rash, hypertension and gastroesophageal reflux disease (GERD)-like esophagitis/gastritis. In most cases, this GERD-like esophagitis/gastritis is self-limited, although some reports exist regarding severe GERD with upper gastrointestinal bleeding (UGIB). We report two cases of men who experienced severe GERD with UGIB while undergoing treatment with sunitinib (Sutene: Pfizer, New York, NY, USA).
The patient was a 70-year-old man who was experiencing bloody vomiting and epigastric pain. He had been diagnosed with high grade clear cell renal carcinoma with lung metastasis in January 2009 and was treated with left radical nephrectomy and underwent chemotherapy until November 2009. However, his metastatic lung nodules were not changed; therefore, sunitinib was started as a standard regimen (50 mg/day for 4 weeks every 6 weeks). On the 10th day of the second cycle of sunitinib, he suffered from heartburn, epigastric pain, and diarrhea, but his symptoms were well controlled with an antisecretory agent and mucosal protector. He took no other medications, such as non steroid anti-inflammatory agents or pain medications that could cause gastroparesis. At the 4 th cycle, he complained of bloody vomiting. Emergency endoscopic examination revealed reflux esophagitis, Los Angeles classification (LA)-C, with acute hyperemic changes (Fig. 1).
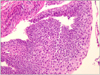
A biopsy from the esophagus showed mucosal ulceration with the formation of granulation tissue, necrosis, and polymorphonuclear leukocyte (Fig. 2A). The adjacent squamous mucosa showed cellular atypia such as hyperchromasia, increases in nuclear size, and multinucleation (Fig. 2B). The patient's symptoms and bleeding improved with treatment with a proton pump inhibitor.
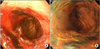
A 56-year-old man presented with nausea and bloody vomiting. He had undergone right nephrectomy and left pulmonary wedge resection in 2007 because of metastatic RCC. He had undergone chemotherapy with sunitinib 50 mg once daily since then. He complained of epigastric pain but had not taken any medication other than sunitinib. The patient was admitted with bloody vomiting in September 2009. Upper endoscopy showed a bleeding esophageal ulceration with underlying reflux esophagitis with hiatal hernia (Fig. 3). A biopsy showed mucosal ulceration covered with fibroinflammatory exudates. The surrounding squamous tissue showed basal cell hyperplasia with increased mitotic figures and infiltration of inflammatory cells. Some of the epithelial cells revealed enlarged and irregularly hyperchromatic nuclei (Fig. 4). He was treated with a proton pump inhibitor and conservative management and sunitinib was discontinued. One month later, his symptoms were improved.
After two months, upper endoscopy showed improvement of the esophageal ulcer but revealed reflux esophagitis, Los-Angeles classification (LA)-D.
Sunitinib, an oral multi-targeted tyrosine kinase inhibitor, acts as an inhibitor of the receptors for vascular endothelial growth factor (VEGF) and platelet-derived growth factor. Sunitinib has demonstrated effects such as reductions in tumor cell proliferation, increased apoptosis, reduced tissue invasion, and reduced angiogenesis [1-3].
Patients treated with either sorafenib and sunitinib have complained of gastroesophageal reflux symptoms, which are detected by endoscopy; however, this occurs more often as a functional irritation of these mucosal surfaces, without macroscopic alterations [4]. A high number of patients complain of swallowing problems due to a burning pain very similar to that reported by patients with GERD [4]. This may ultimately lead to anorexia/cachexia in advanced cancer patients.
The pathogenesis of this adverse event is still not known. We hypothesized that both VEGF and the mitogen activated protein kinase pathway are involved in the process of mucosal defense and repair following acid-induced injury. Therefore, the inhibition of these pathways could lead to an enhanced sensitivity to irritation caused by peptic acid secretion, at both the esophageal and gastric levels [4].
The proteins of antiangiogenic inhibitors, as a marker of biological activity, were mainly expressed as cellular atypia of squamous mucosa. The histopathologic findings of the esophagus are considered to occur in response to injury from gastroesophageal reflux. The cellular atypia of squamous mucosa of these two cases appeared to be associated with reparative processes. This supports the idea that the anti-angiogenesis activity induced by sunitinib in acid damaged distal esophagus might be the main pathogenesis of the GERD-like esophagitis.
In conclusion, we reported two cases of sunitinib-related complicated reflux esophagitis, which were supported by specific pathologic changes. No previous studies have reported sunitinib related severe GERD, such as upper gastrointestinal bleeding. The use of sunitinib is rapidly growing [5], which means that increased knowledge about its side effects-as well as proactive assessment and consistent management of sunitinib related side effects will be critical to ensure optimal benefits from sunitinib treatment.
Figures and Tables
Fig. 1
Endoscopic finding. Linear mucosal breaks are noted at the distal esophagus, with some confluence.
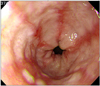
Fig. 2
Microscopic finding. (A) Mucosa show granulation tissue covered with necroinflammatory exudates due to ulceration (H&E, ×100). (B) The squamous epithelial cells reveal enlarged, hyperchromatic nuclei with a few multinucleations (H&E, ×100).

References
1. Chow LQ, Eckhardt SG. Sunitinib: from rational design to clinical efficacy. J Clin Oncol. 2007. 25:884–896.
2. Atkins M, Jones CA, Kirkpatrick P. Sunitinib maleate. Nat Rev Drug Discov. 2006. 5:279–280.
3. Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007. 356:115–124.
4. Porta C, Paglino C, Imarisio I, Bonomi L. Uncovering. Clin Exp Med. 2007. 7:127–134.
5. Di Lorenzo G, Buonerba C, Biglietto M, Scognamiglio F, Chiurazzi B, Riccardi F, et al. The therapy of kidney cancer with biomolecular drugs. Cancer Treat Rev. 2010. 36:Suppl 3. S16–S20.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download