Abstract
Amiodarone has been widely used for supraventricular and ventricular arrhythmias and many patients benefit from its effectiveness in treating potentially life-threatening arrhythmias. However, this drug can cause multi-organ toxicity, including amiodarone-induced pulmonary toxicity (APT). Not only does amiodarone have a long half-life but also is lipophilic and therefore can easily accumulate in tissues. Hence, it is difficult to monitor therapeutic levels and side effects, making it difficult to predict toxicities. In this case, we describe multi-organ complications secondary to amiodarone use, especially APT combined with pneumonia with atypical pathogens and pulmonary hemorrhage. The patient reached a high cumulative dose of amiodarone despite a low maintenance dose of amiodarone. This case highlights an unusual presentation of APT with multi-organ toxicity and we review articles regarding the association between the cumulative dose of amiodarone and amiodarone-induced toxicities.
Amiodarone, a bi-iodinated benzofuran derivative, is widely used for arrhythmias. Many patients benefit from the effectiveness of amiodarone in treating potentially life-threatening arrhythmias. However, amiodarone-induced pulmonary toxicity (APT) is a severe complication. Even after the discontinuation of amiodarone, not only is APT difficult to manage, but there is also a potentially life-threatening risk of recurrence of arrhythmias. In this case, we describe multiorgan complications secondary to amiodarone use, especially APT combined with pneumonia with atypical pathogens and pulmonary hemorrhage following procedures. Following treatment and stabilization, the patient experienced sudden cardiac death while recovering from pulmonary complications.
A 65-year-old man presented with fever, worsening cough, and dyspnea. He had lost 10 kg over the past 2 months. He had a medical history of atrial fibrillation; myocardial infarction caused by diffuse spasm of the left and right coronary arteries; and aborted sudden cardiac death from ventricular tachycardia, which was converted to sinus rhythm following electric cardioversion. He was a 40 pack-year smoker and a chronic alcoholic. Regular medications included aspirin, nicorandil, diltiazem, valsartan, warfarin, and amiodarone. He had taken 400 mg/day amiodarone for 3 years (a total cumulative dose of 232 g) to control atrial fibrillation and life-threatening ventricular tachycardia. Because APT was suspected, amiodarone was immediately discontinued. On examination, the patient had a regular heart rate of 78 beats/min, blood pressure of 90/50 mm Hg, respiratory rate of 20 breaths/min, and body temperature of 36.2℃. Breath sounds were clear without crackles or wheezing and heart sounds were regular with normal S1, S2 sound without murmur. Initial electrocardiography indicated normal sinus rhythm. Carbon monoxide diffusion in the lung was decreased at 11.4 mL·mm Hg-1min-1 (64% of the predictive value; reference 17.8 mL·mm Hg-1min-1). Laboratory results were as follows: potassium level, 4.6 mmol/L (normal range, 3.5 to 5.0 mmol/L); alanine transaminase level, 66 U/L (normal range, ≤33 U/L), aspartate transaminase level, 74 U/L (normal range, <32 U/L); creatinine level, 1.2 mg/dL (normal range, 0.6 to 1.2 mg/dL); C-reactive protein level, 23.32 mg/dL (normal range, 0 to 0.3 mg/dL); and erythrocyte sedimentation rate, 120. Chest radiography showed new consolidations in the right upper lobe and reticulonodular opacities in both lung fields compared with previous normal chest radiography (Fig. 1). Chest computed tomography (CT) revealed multifocal subpleural consolidations with high attenuation in parenchymal lesions in both lungs, which had a bronchiolitis obliterans obstructive pneumonia pattern, as well as increased attenuation of the liver, suggestive of amiodarone exposure (Fig. 2). On the basis of the chest CT findings, combined pneumonia or tuberculosis infection was also suspected. Fungus (Aspergillus) and methicillin-resistant Streptococcus aureus were isolated from sputum. Parenteral amphotericin and teicoplanin were prescribed for pneumonia. After discontinuing warfarin and aspirin for 7 days, percutaneous needle aspiration biopsy (PCNB) was performed at the right upper lobe lesion for differential diagnosis of atypical pathogens, amiodarone induced lung injury and malignancy. Immediately after PCNB, the patient had hemoptysis of approximately 20~30 mL. After stabilization with supportive care including intravenous injection of vitamin K and transfusion of fresh frozen plasma, aspirin and warfarin were resumed.
The patient then complained of general weakness, and objectively, his muscle strength was slightly decreased. Thyroid function tests were abnormal: the triiodothyronine (T3) level was normal at 73.3 ng/dL (reference range, 58 to 159 ng/dL); the thyroxine (fT4) level was increased at 2.64 ng/dL (reference range, 0.7 to 1.48 ng/dL); and the thyroid stimulating hormone level was decreased at 0.03 µIU/mL (reference range, 0.35 to 4.94 µIU/mL). The thyroglobulin antibody level was markedly elevated at 206.9 IU/mL (reference range, <115 IU/mL). A thyroid scan revealed decreased 24-hour iodine uptake (Fig. 3). These results suggested amiodarone-induced thyrotoxicosis without hyperthyroidism.
Mycobacterium avium was isolated from an initial sputum culture; however the patient was not on antituberculosis medications beside macrolide for any possible atypical pathogens. Six days after resuming aspirin and warfarin, the patient experienced massive hemoptysis with acute respiratory distress syndrome (ARDS), requiring endotracheal intubation, artificial ventilator support, and urgent bronchial artery embolization. However, ARDS failed to improve despite the management. Pathologic analysis of PCNB sample showed chronic interstitial pneumonia with fibrous thickening of alveolar septa, chronic inflammation, and collections of intra-alveolar foamy macrophages in alveolar spaces, which were positive on Oil-Red-O staining (Fig. 4). The ultrastructure of foamy macrophages revealed numerous lysosomal inclusion bodies, which had electron-dense multilamellated deposits (Fig. 5). These histological findings were consistent with those of amiodarone-induced toxicities.
After the administration of high dose parenteral steroids, ARDS gradually improved. The patient was successfully weaned from the artificial ventilator and his general condition continued to improve. However, he faced sudden cardiac death, which was probably caused by a ventricular arrhythmia.
Amiodarone is similar in structure to the thyroid hormone. It is lipophilic and therefore can easily accumulate in tissues. Further, amiodarone has a long half-life of more than 50 days. Hence, it is difficult to monitor therapeutic levels and side effects, and toxicities are unpredictable.
Amiodarone toxicity has been reported to affect the liver, lung, eye, heart, thyroid, nerves, and other organs [1]. In particular, APT can present as interstitial pneumonia, lung fibrosis, various types of pneumonia, and life-threatening ARDS. The incidence of APT is approximately 5~10%. The risk factors for APT remain unclear, but advanced age, pre-existing lung disease, and past thoracotomy are regarded as risk factors [2,3].
Studies have found no association between the dose of amiodarone (both initial loading and maintenance dose) and APT [2]. For example, patents who have taken a low dose of amiodarone or those who have taken amiodarone for less than 1 year frequently experienced APT [4,5]. Some authors recommend using a low maintenance dose of < 400 mg/day because of the relatively low likelihood of developing APT at this dose [3,6].
However, many recent studies have found that a high cumulative dose of amiodarone is closely associated with pulmonary and thyroid toxicities, even with low maintenance doses of amiodarone (1,7,8). One study reported that long-term use of a low maintenance dose of amiodarone in the elderly, with a cumulative high total dose of 101~151 g, increased the risk of APT significantly (odds ratio [OR], 10.29; 95% confidence interval [CI], 3.42 to 30.92) and reached a plateau at the cumulative dose of >150 g (OR, 9.5; 95% CI 3.8 to 23.67) [7]. Similarly, another study reported a significantly increased ATP risk with cumulative doses of 140~230 mg [9]. In this case, a low maintenance dose of 400 mg/day amiodarone for 3 years was prescribed, which was quite a long period. As the result, the patient's total cumulative dose was > 200 g. Although there were no pathognomonic findings for APT, pathology results from the needle biopsy showed foamy macrophages with characteristic lysosomal inclusions and typical electron-dense multilamellar bodies. APT is a diagnosis of exclusion and frequent manifest as chronic interstitial pneumonitis and pulmonary fibrosis [2,3]. Given the patient's medical history, clinical presentation, pathologic findings, and especially the rapid improvement with parenteral high-dose steroids, the diagnosis of APT was clear. However, this case had some complex components. The patient was a 40 pack-year current smoker and also suffered from lung infections caused by multiple atypical organisms, so multimodal therapy was important. When APT was suspected, the first step was to discontinue amiodarone and use another antiarrhythmic therapy while managing APT. The second step was to address and treat other possible causes.
Another rare presentation of APT is pulmonary alveolar hemorrhage [10]. Although the patient in this case experienced hemoptysis, the first episode occurred after PCNB and PCNB pathology did not show evidence of hemophagocytic macrophages. The second episode of massive hemoptysis occurred 6 days after aspirin and warfarin treatment was resumed. Following the massive hemoptysis, the patient developed ARDS, which was controlled by bronchial artery embolization and high-dose parenteral steroids. The patient's overall respiratory condition rapidly improved, not because of antibiotic treatment for the atypical pneumonia, but rather in response to high-dose steroid therapy. The association between hemoptysis and APT remains questionable in this case.
The cumulative dose of amiodarone is also important in the development of thyroid toxicities. One study showed that the risk of thyroid dysfunction increases with an increasing cumulative dose, and when the cumulative dose is >144 g, the adjusted OR is 12.0 (95% CI, 6.1 to 27.3) [8]. Amiodarone-induced hyperthyroidism was also noted in this case. New thyroid dysfunction was confirmed by laboratory tests, and a thyroid scan showed decreased 24-hour iodine uptake.
Several mechanisms of amiodarone toxicities have been proposed, but there are 2 major basic mechanisms [9,10]. The first mechanism constitutes an immune-mediated hypersensitivity and the second is a direct drug-induced phospholipidosis. Dose-independent acute onset toxicities usually have a rapidly progressive course and can be unpredictable. This pattern might be related to APT, which occurs after initial or low-dose amiodarone use. While, insidious onset toxicities commonly progress more slowly than immune-mediated hypersensitivity and typically present in patients who have taken amiodarone for > 2 months [10]. Common symptoms include weight loss, chronic aggravating cough, and dyspnea, conditions that were apparent in the clinical presentation of this patient.
This case reiterates the importance of the cumulative dose of amiodarone. Physicians should carefully evaluate patients who have been taking low maintenance doses of amiodarone for a long time, even if they are asymptomatic. This is particularly important if the cumulative dose is >100 g, as the possibilities of lung and thyroid toxicities should be weighed. In these cases, we recommend that chest CT, pulmonary function tests, thyroid function tests, and serial follow-up of liver enzymes be performed.
Figures and Tables
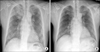 | Fig. 1Chest X-ray showing new right upper lobe consolidation with reticulonodular opacities on both lung fields (A), when compared to a previous chest X-ray (B). |
 | Fig. 2Chest computed tomography scan showing subpleural consolidation with internal high attenuation (A), subpleural reticular opacity and interlobular septal thickening with patchy ground-glass opacities (B), and diffuse increase in the attenuation of the liver (C). |
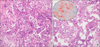 | Fig. 4Pathologic analysis of percutaneous needle aspiration biopsy sample showing chronic interstitial inflammation with fibrous thickening of alveolar septum with features of organizing pneumonia (A: H&E, ×200). Some alveolar spaces showing collection of many intra-alveolar foamy macrophages (B: H&E, ×400). A circle shows Oil Red O-positive lipid droplets in the alveolar macrophages. |
References
1. Stelfox HT, Ahmed SB, Fiskio J, Bates DW. Monitoring amiodarone's toxicities: recommendations, evidence, and clinical practice. Clin Pharmacol Ther. 2004; 75:110–122.
2. Jackevicius CA, Tom A, Essebag V, Eisenberg MJ, Rahme E, Tu JV, et al. Population-level incidence and risk factors for pulmonary toxicity associated with amiodarone. Am J Cardiol. 2011; 108:705–710.
3. Adams GD, Kehoe R, Lesch M, Glassroth J. Amiodarone-induced pneumonitis: assessment of risk factors and possible risk reduction. Chest. 1988; 93:254–263.
4. Jang WJ, Chon HR, Jung JS, Yoo SH, Koh KH, Koh YM, et al. Amiodarone-induced pulmonary toxicity within a short period of the initiation of amiodarone therapy: a case report. Korean J Crit Care Med. 2011; 26:117–121.
5. Veltri EP, Reid PR. Amiodarone pulmonary toxicity: early changes in pulmonary function tests during amiodarone rechallenge. J Am Coll Cardiol. 1985; 6:802–805.
6. Kerin NZ, Rubenfire M. Detection of amiodarone pulmonary toxicity. J Am Coll Cardiol. 1989; 13:261–262.
7. Ernawati DK, Stafford L, Hughes JD. Amiodarone-induced pulmonary toxicity. Br J Clin Pharmacol. 2008; 66:82–87.
8. Bouvy ML, Heerdink ER, Hoes AW, Leufkens HG. Amiodarone-induced thyroid dysfunction associated with cumulative dose. Pharmacoepidemiol Drug Saf. 2002; 11:601–606.
9. Ashrafian H, Davey P. Is amiodarone an underrecognized cause of acute respiratory failure in the ICU? Chest. 2001; 120:275–282.
10. Tanawuttiwat T, Harindhanavudhi T, Hanif S, Sahloul MZ. Amiodarone-induced alveolar haemorrhage: a rare complication of a common medication. Heart Lung Circ. 2010; 19:435–437.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download